SKIN CRAVE ALL NATURAL SPF30- all natural spf30 sunscreen lotion lotion
Tropical Enterprises International, Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
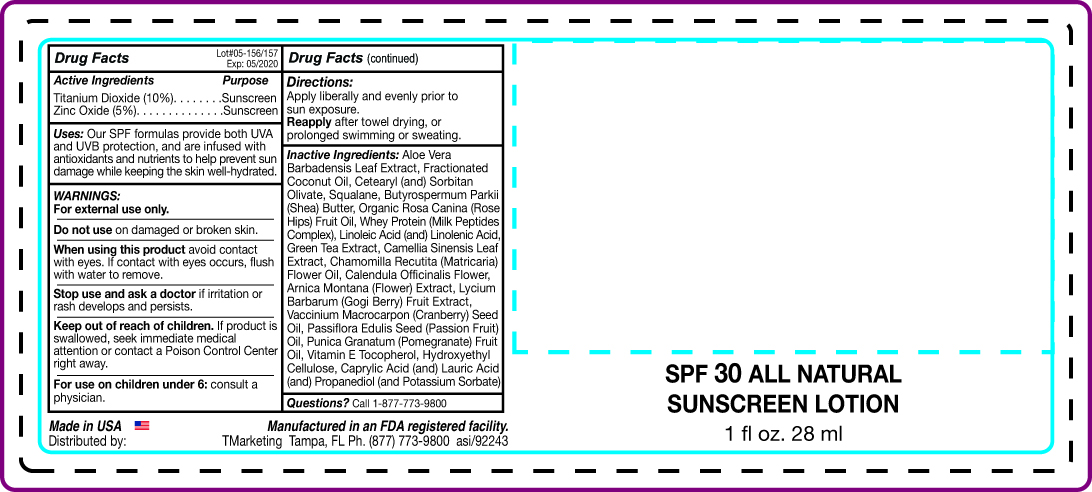
All Natural Spf30 Broad Spectrum Sunscreen - AN
Uses: Our SPF formulas provide both UVA and UVB protection, and are infused with antioxidants
and nutrients to help prevent sun damage while keeping the skin well-hydrated.
When using this product avoid contact
with eyes. If contact with eyes occurs, flush with water to remove.
Keep out of reach of children. If product is
swallowed, seek immediate medical
attention or contact a Poison Control Center
right away.
Directions:
Apply liberally and evenly prior to sun exposure.
Reapply after towel drying, or prolonged swimming or sweating.
Inactive Ingredients: Aloe Vera
Barbadensis Leaf Extract, Fractionated
Coconut Oil, Cetearyl (and) Sorbitan Olivate, Squalane,
Butyrospermum Parkii (Shea) Butter, Organic Rosa Canina
(Rose Hips) Fruit Oil, Whey Protein (Milk Peptides Complex), Linoleic Acid
(and) Linelenic Acid, Green Tea Extract, Camellia Sinensis Leaft Extract, Cjamomilla Recutita
(Matricaria) Flower Oil, Calendula Officinalis Flower, Arnica Montana (Flower) Extract, Lycium
Barbarum (Gogi Berry) Fruit Extract, Vaccinium Macrocarpon (Cranberry) Seed Oil, Passiflora
Edulis Seed (Passion Fruit) Oil, Vitamin E Tocopherol, Hydroxyethyl Cellulose, Caprylic Acid
(and) Lauric Acid (and) Propanediol (and Potassium Sorbate)
| SKIN CRAVE ALL NATURAL SPF30
all natural spf30 sunscreen lotion lotion |
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Tropical Enterprises International, Inc (091986179) |
| Registrant - Tropical Enterprises International, Inc. (091986179) |