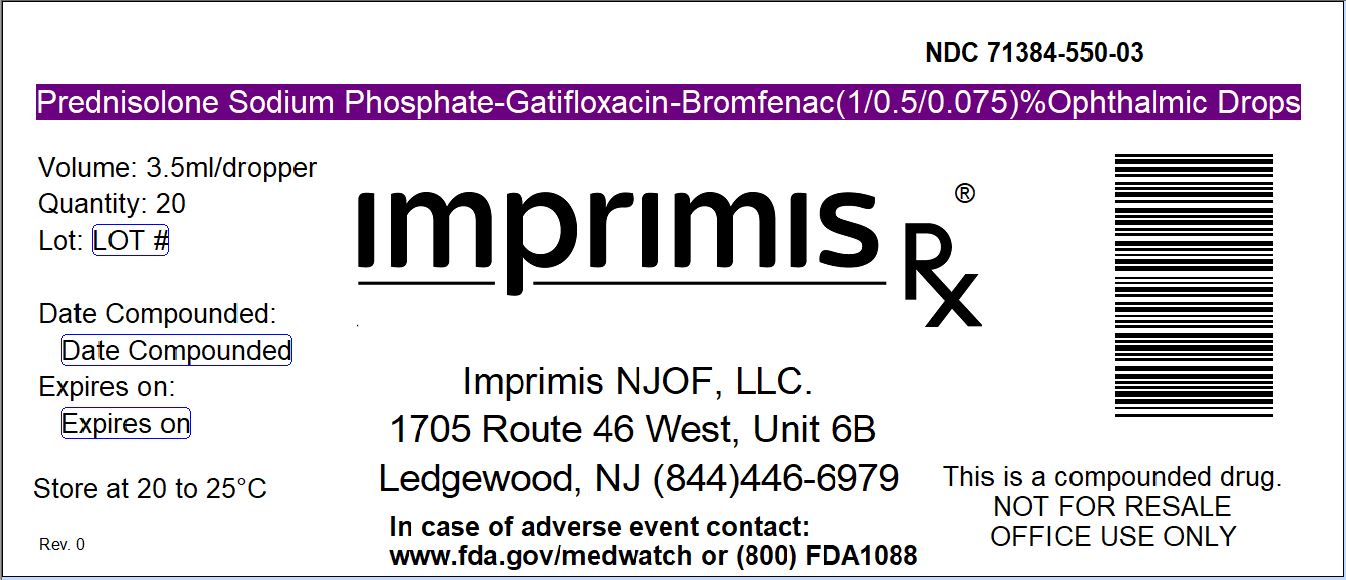
PRED PHOS-GATI-BROM- prednisolone phosphate - gatifloxacin - bromfenac solution/ drops
Imprimis NJOF, LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
| PRED PHOS-GATI-BROM
prednisolone phosphate - gatifloxacin - bromfenac solution/ drops |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Imprimis NJOF, LLC (080431967) |
Revised: 2/2020
Document Id: 9e644f55-0f3c-04b8-e053-2a95a90ab300
Set id: 6dd75810-da95-28aa-e053-2a91aa0a0dab
Version: 3
Effective Time: 20200212
Imprimis NJOF, LLC