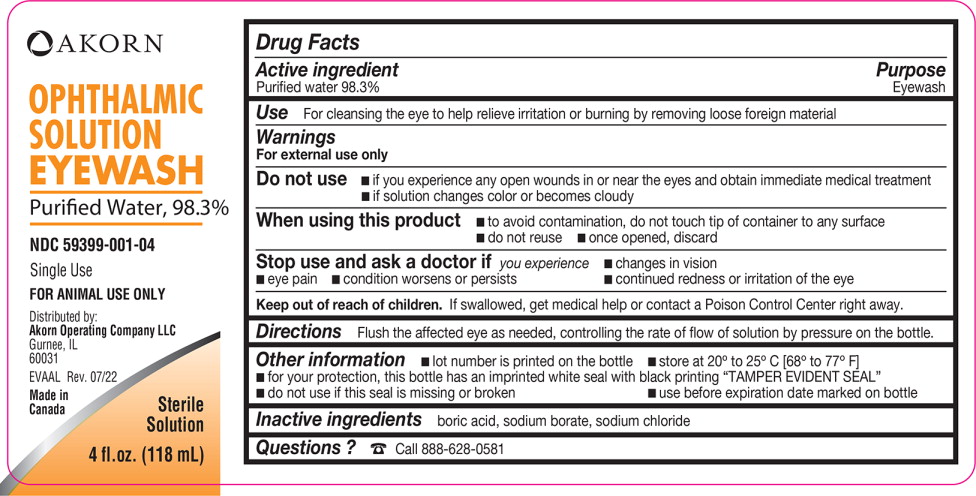
Label: EYEWASH- water solution
- NDC Code(s): 59399-001-04, 59399-001-08
- Packager: Akorn
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated July 12, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
-
Warnings
For external use only
Do not use
- if you experience any open wounds in or near the eyes and obtain immediate medical treatment
- if solution changes color or becomes cloudy
When using this product
- to avoid contamination, do not touch tip of container to any surface
- do not reuse
- once opened, discard
- Directions
- Other information
- Inactive ingredients
- Questions ?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EYEWASH
water solutionProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:59399-001 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Water (UNII: 059QF0KO0R) (Water - UNII:059QF0KO0R) Water 929 g in 946 mL Inactive Ingredients Ingredient Name Strength Boric Acid (UNII: R57ZHV85D4) Sodium Borate (UNII: 91MBZ8H3QO) Sodium Chloride (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59399-001-04 1 in 1 BOTTLE 1 118 mL in 1 BOTTLE 2 NDC:59399-001-08 1 in 1 BOTTLE 2 236 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug other 02/01/2015 Labeler - Akorn (117693100)