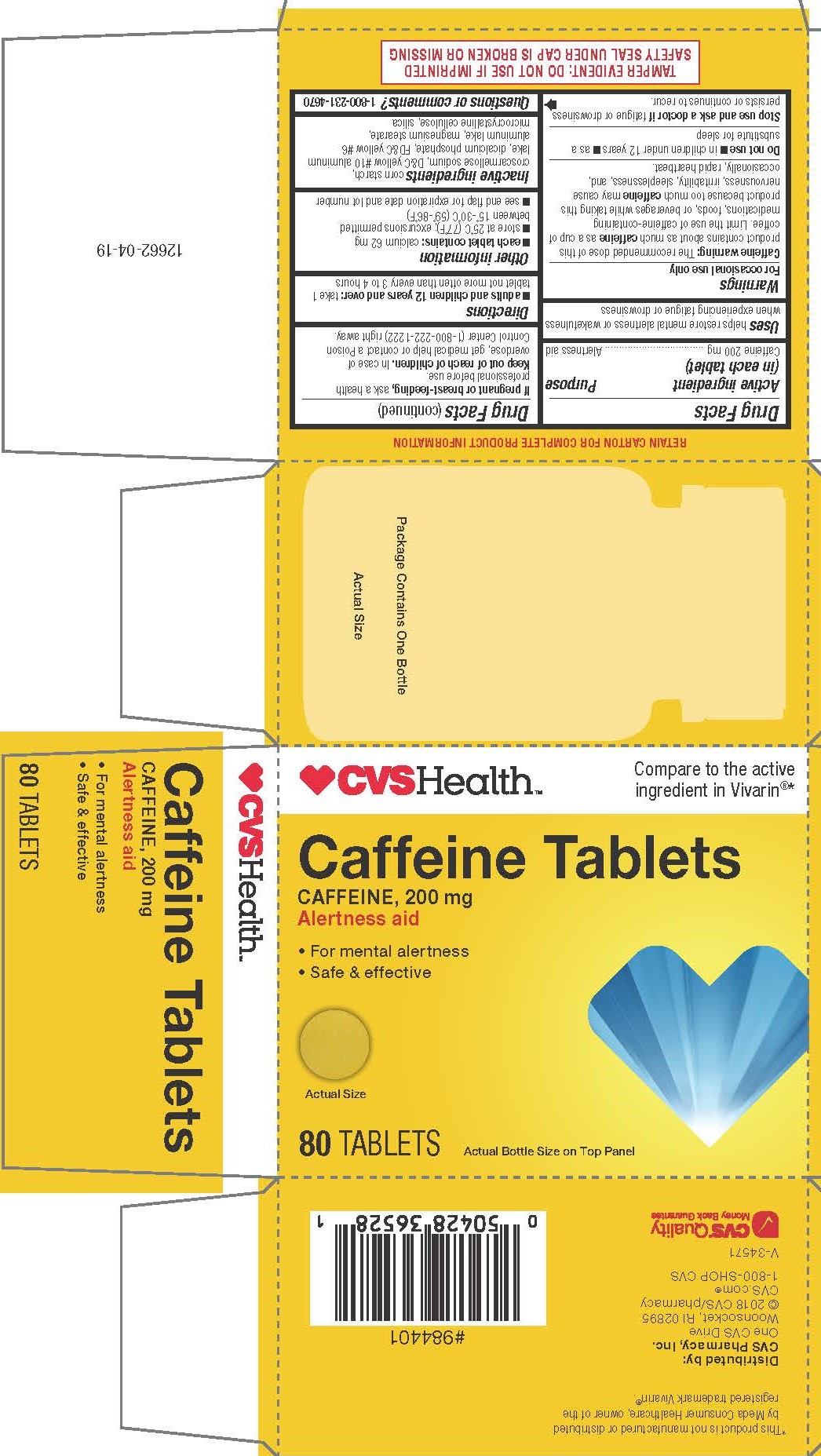
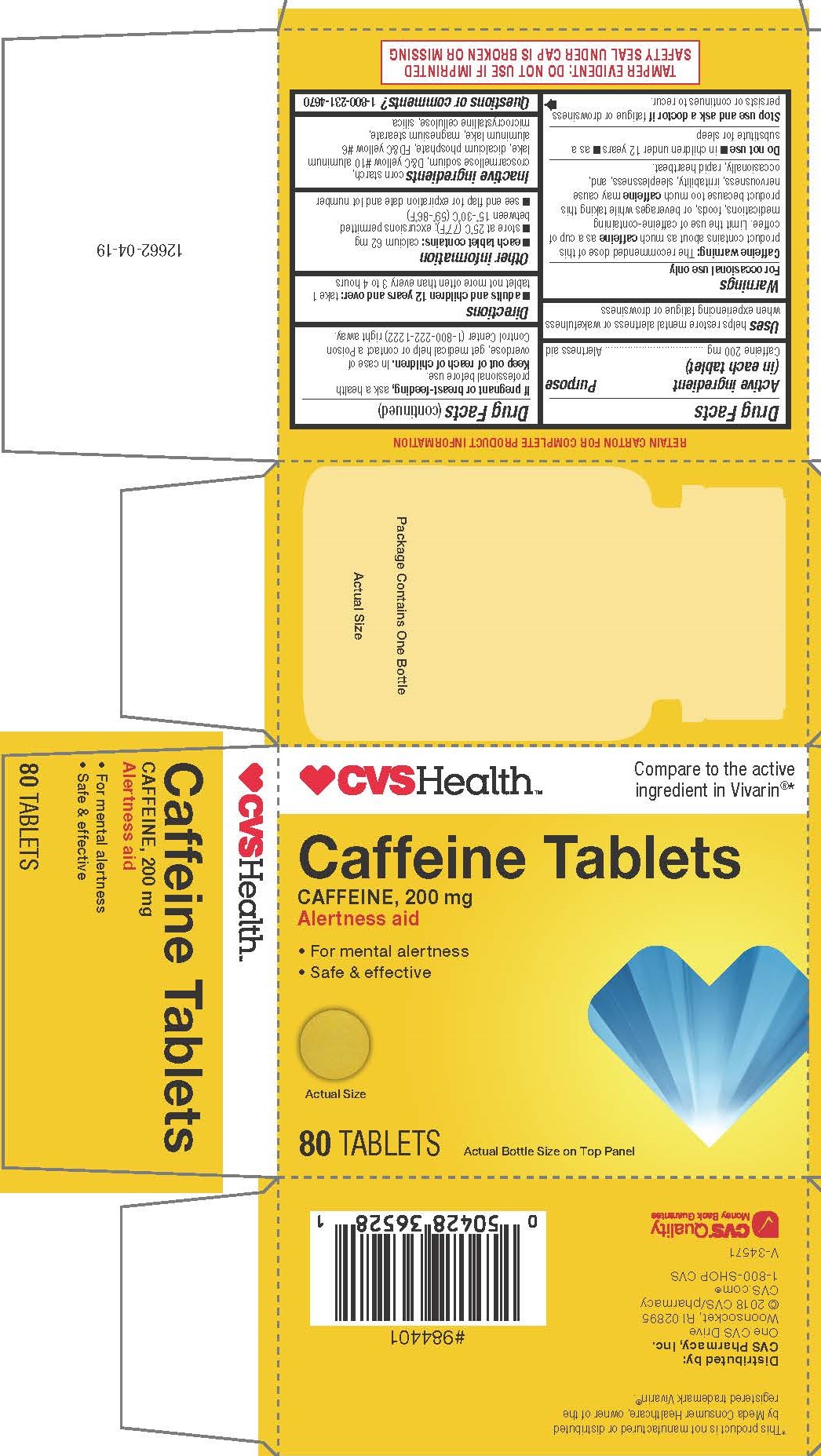
Label: CAFFEINE tablet
- NDC Code(s): 69842-509-10, 69842-509-21, 69842-509-31
- Packager: CVS Pharmacy, Inc.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 23, 2020
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- PURPOSE
- Uses
-
WARNINGS
For occasional use only
Caffeine warning: The recommended dose of this product contains about as much caffeine as a cup of coffee. Limit the use of caffeine-containing medications, foods, or beverages while taking this product because too much caffeine may cause nervousness, irritability, sleeplessness, and, occasionally, rapid heartbeat.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
SPL UNCLASSIFIED SECTION
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
*This product is not manufactured or distributed by Meda Consumer Healthcare, owner of the registered trademark Vivarin®.
Distributed by:
CVS Pharmacy, Inc.
One CVS Drive
Woonsocket, RI 02895
© 2018 CVS/pharmacy
CVS.com®
1-800-SHOP CVS
V-34571
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CAFFEINE
caffeine tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69842-509 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAFFEINE (UNII: 3G6A5W338E) (CAFFEINE - UNII:3G6A5W338E) CAFFEINE 200 mg Inactive Ingredients Ingredient Name Strength D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) STARCH, PREGELATINIZED CORN (UNII: O8232NY3SJ) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) MAGNESIUM STEARATE (UNII: 70097M6I30) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) Product Characteristics Color yellow Score no score Shape ROUND Size 11mm Flavor Imprint Code 212;212 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69842-509-10 5 in 1 CARTON 06/22/2018 1 8 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:69842-509-21 2 in 1 CARTON 06/22/2018 2 8 in 1 BLISTER PACK; Type 0: Not a Combination Product 3 NDC:69842-509-31 1 in 1 CARTON 06/22/2018 3 80 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part340 06/22/2018 Labeler - CVS Pharmacy, Inc. (062312574)