Label: PATADAY ONCE DAILY RELIEF- olopatadine hydrochloride solution
- NDC Code(s): 0065-0816-01, 0065-0816-02, 0065-0816-04, 0065-0816-12
- Packager: Alcon Laboratories, Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated June 21, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INDICATIONS & USAGE
- WARNINGS
- Do not use
- When using this product
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
Directions
-
adults and children 2 years of age and older:
- put 1 drop in the affected eye(s) once daily
-
do not use more than 1 drop in each eye per day
- if using other ophthalmic products while using this product, wait at least 5 minutes between each product
- replace cap after each use
- children under 2 years of age: consult a doctor
-
adults and children 2 years of age and older:
- Other information
- Inactive ingredients
- Questions?
-
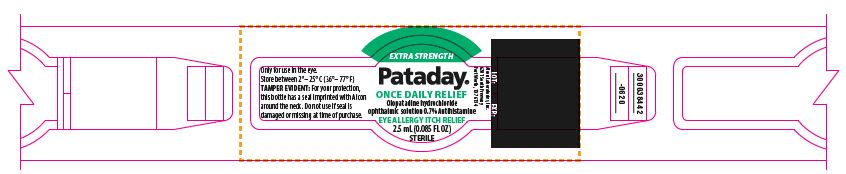
PRINCIPAL DISPLAY PANEL
EXTRA STRENGTH
Pataday®
ONCE DAILY RELIEF
Olopatadine hydrochloride
ophthalmic solution 0.7%
Antihistamine
Eye Allergy Itch Relief
FULL 24 HOUR
Works in Minutes
Relief from Allergens:
• Pet Dander
• Pollen
• Grass
• Ragweed
Alcon
STERILE
2.5 mL (0.085 FL OZ)
EXTRA STRENGTH
Pataday®
ONCE DAILY RELIEF
TAMPER EVIDENT: For your protection, this bottle has a seal imprinted with Alcon around the neck. Do not use if seal is damaged or missing at time of purchase.
30 DAY SUPPLY
Eye Allergy Itch Relief
Works in Minutes
For Ages 2 and Older
________Fill Line________
Alcon Laboratories, Inc.
6201 South Freeway
Fort Worth, Texas 76134
Country of Origin: Japan
ACTUAL SIZE
NDC: 0065-0816-04
300038456-0620
LOT: EXP.:
3187

EXTRA STRENGTH
Pataday®
ONCE DAILY RELIEF
Olopatadine hydrochloride
ophthalmic solution 0.7% Antihistamine
2.5 mL (0.085 FL OZ)
STERILE
Only for use in the eye.
Store between 2°– 25° C (36°– 77° F)
TAMPER EVIDENT: For your protection, this bottle has a seal imprinted with Alcon around the neck. Do not use if seal is damaged or missing at time of purchase.
Alcon Laboratories, Inc.
6201 South Freeway
Fort Worth, TX 76134
LOT: EXP.:
300038442-0620
-
INGREDIENTS AND APPEARANCE
PATADAY ONCE DAILY RELIEF
olopatadine hydrochloride solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0065-0816 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Olopatadine Hydrochloride (UNII: 2XG66W44KF) (Olopatadine - UNII:D27V6190PM) Olopatadine 7 mg in 1 mL Inactive Ingredients Ingredient Name Strength Benzalkonium Chloride (UNII: F5UM2KM3W7) Boric Acid (UNII: R57ZHV85D4) Hydrochloric Acid (UNII: QTT17582CB) Sodium Hydroxide (UNII: 55X04QC32I) Hydroxypropyl .Gamma.-Cyclodextrin (UNII: P6BYU725IU) Hypromellose, Unspecified (UNII: 3NXW29V3WO) Mannitol (UNII: 3OWL53L36A) Polyethylene Glycol 400 (UNII: B697894SGQ) Povidone, Unspecified (UNII: FZ989GH94E) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0065-0816-04 1 in 1 CARTON 09/01/2020 1 2.5 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC:0065-0816-01 2 in 1 CARTON 01/04/2021 2 2.5 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 3 NDC:0065-0816-02 1 in 1 CARTON 01/04/2021 3 0.5 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 4 NDC:0065-0816-12 3 in 1 CARTON 01/24/2022 4 2.5 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA206276 09/01/2020 Labeler - Alcon Laboratories, Inc. (008018525) Establishment Name Address ID/FEI Business Operations Alcon Research LLC 007672236 manufacture(0065-0816)