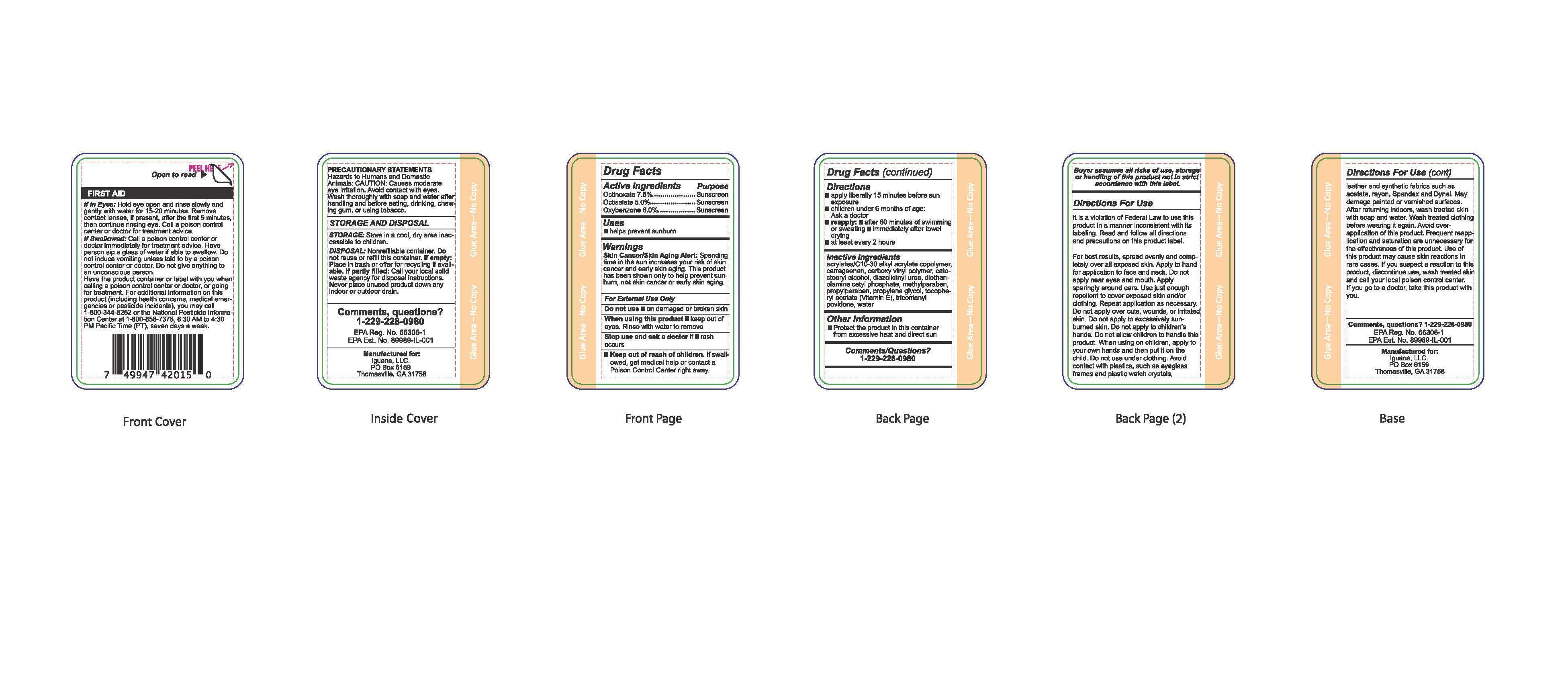
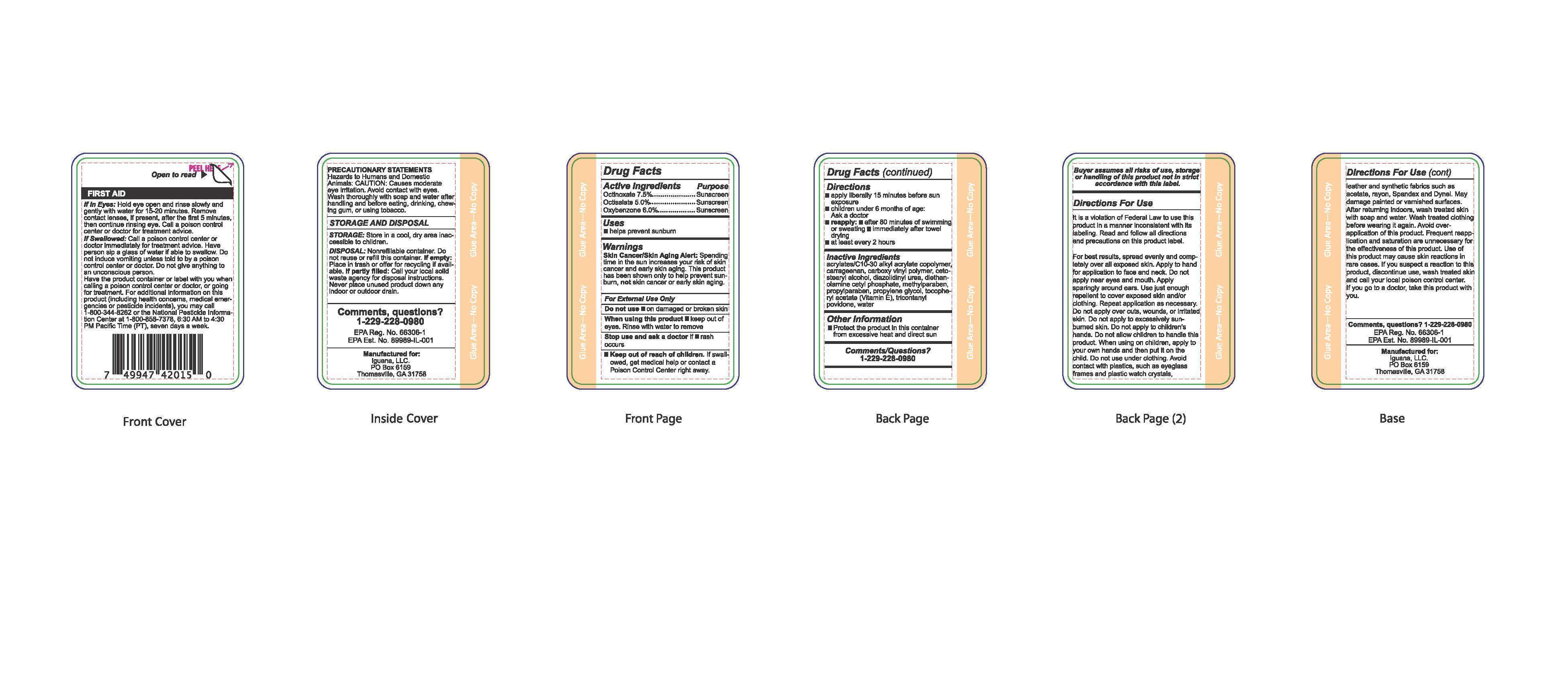
Label: SUNSECT INSECT REPELLENT SUNSCREEN- octinoxate, oxybenzone, octisalate lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 57595-001-01, 57595-001-02, 57595-001-03, 57595-001-04 - Packager: Iguana LLC
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 20, 2015
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
-
Warnings
Skin Cancer/Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to help prevent sunbrun, not skin cancer or early skin aging.
For external use only. Do not use on damaged or broken skin. When using this product keep out of eyes. Rinse with water to remove. Stop use and ask a doctor if rash occurs. Keep out of the reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
- Directions
- Other Information
- Inactive Ingredients
- Comments/Questions?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
SUNSECT INSECT REPELLENT SUNSCREEN
octinoxate, oxybenzone, octisalate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57595-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 g in 100 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 6 g in 100 g DIETHYLTOLUAMIDE (UNII: FB0C1XZV4Y) (DIETHYLTOLUAMIDE - UNII:FB0C1XZV4Y) DIETHYLTOLUAMIDE 20 g in 100 g Inactive Ingredients Ingredient Name Strength CARBOMER COPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 809Y72KV36) CARBOMER HOMOPOLYMER TYPE C (UNII: 4Q93RCW27E) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Carrageenan (UNII: 5C69YCD2YJ) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) DIETHANOLAMINE CETYL PHOSPHATE (UNII: 4UG0316V9S) TRICONTANYL POVIDONE (UNII: N0SS3Q238D) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) WATER (UNII: 059QF0KO0R) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) CITRONELLA OIL (UNII: QYO8Q067D0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57595-001-01 59 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/01/2015 2 NDC:57595-001-02 118 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/01/2015 3 NDC:57595-001-03 3672 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 06/01/2015 4 NDC:57595-001-04 50 in 1 BOX 06/01/2015 4 9 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 06/01/2015 Labeler - Iguana LLC (109358551) Establishment Name Address ID/FEI Business Operations Solo Laboratories, Inc. 005126784 manufacture(57595-001) , pack(57595-001) , label(57595-001)