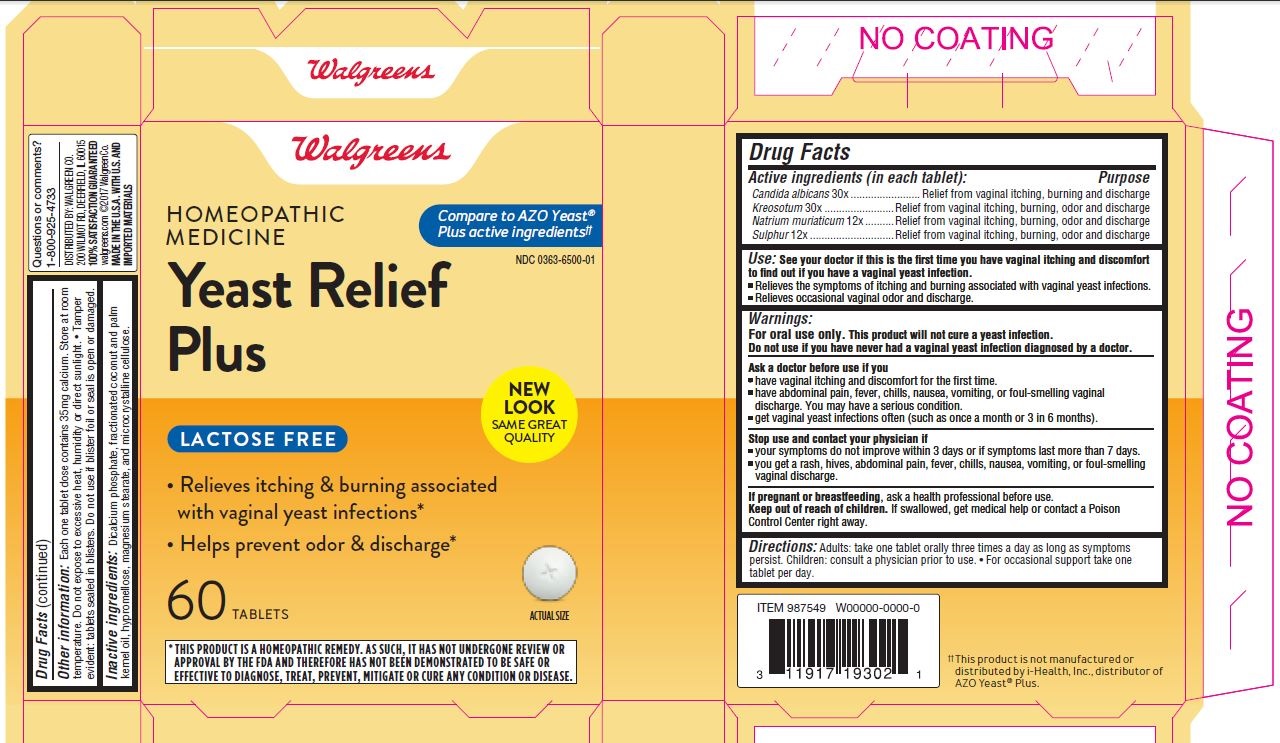
Label: HOMEOPATHIC MEDICINE YEAST RELIEF PLUS- candida albicans, wood creosote, sodium chloride, and sulfur tablet
- NDC Code(s): 0363-6500-01
- Packager: WALGREEN CO.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated November 30, 2021
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients (in each tablet):
- Purpose
- Use:
-
Warnings:
For oral use only. This product will not cure a yeast infection.
Do not use if you have never had a vaginal yeast infection diagnosed by a doctor.
Ask a doctor before use if you
- have vaginal itching and discomfort for the first time.
- have abdominal pain, fever, chills, nausea, vomiting, or foul-smelling vaginal discharge. You may have a serious condition.
- get vaginal yeast infections often (such as once a month or three in six months).
- may have been exposed to human immunodeficiency virus (HIV) that causes AIDS.
- DOSAGE & ADMINISTRATION
- OtherInformation:
- Inactive Ingredients:
- GENERAL PRECAUTIONS
- 0363-6500-01 Homeopathic Medicine Yeast Relief Plus
-
INGREDIENTS AND APPEARANCE
HOMEOPATHIC MEDICINE YEAST RELIEF PLUS
candida albicans, wood creosote, sodium chloride, and sulfur tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0363-6500 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CANDIDA ALBICANS (UNII: 4D7G21HDBC) (CANDIDA ALBICANS - UNII:4D7G21HDBC) CANDIDA ALBICANS 30 [hp_X] in 1 g WOOD CREOSOTE (UNII: 3JYG22FD73) (WOOD CREOSOTE - UNII:3JYG22FD73) WOOD CREOSOTE 30 [hp_X] in 1 g SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 12 [hp_X] in 1 g SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 12 [hp_X] in 1 g Inactive Ingredients Ingredient Name Strength CALCIUM PHOSPHATE, DIBASIC, DIHYDRATE (UNII: O7TSZ97GEP) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) MAGNESIUM STEARATE (UNII: 70097M6I30) HYPROMELLOSES (UNII: 3NXW29V3WO) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) Product Characteristics Color white Score no score Shape ROUND Size 10mm Flavor Imprint Code Plus Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0363-6500-01 60 in 1 BOX 05/18/2017 1 0.375 g in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/18/2017 Labeler - WALGREEN CO. (008965063)