Label: RAINFOREST OF THE SEA WATER FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 8S PORCELAIN- titanium dioxide and zinc oxide liquid
RAINFOREST OF THE SEA WATER FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 12B FAIR BEIGE- titanium dioxide and zinc oxide liquid
RAINFOREST OF THE SEA WATER FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 12N FAIR NEUTRAL (titanium dioxide and zinc oxide) .......ng> RAINFOREST OF THE SEA WATER FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 57N RICH NEUTRAL (titanium dioxide and zinc oxide) liquid RAINFOREST OF THE SEA WATER FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 57S RICH SAND (titanium dioxide and zinc oxide) liquid RAINFOREST OF THE SEA WATER FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 61H MAHOGANY- titanium dioxide and zinc oxide liquid [Tarte, Inc]
RAINFOREST OF THE SEA WATER FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 57N RICH NEUTRAL- titanium dioxide and zinc oxide liquid
RAINFOREST OF THE SEA WATER FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 57S RICH SAND- titanium dioxide and zinc oxide liquid
RAINFOREST OF THE SEA WATER FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 61H MAHOGANY- titanium dioxide and zinc oxide liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 51060-167-01, 51060-168-01, 51060-169-01, 51060-170-01, view more51060-171-01, 51060-172-01, 51060-173-01, 51060-174-01, 51060-175-01, 51060-176-01, 51060-177-01, 51060-178-01, 51060-179-01, 51060-180-01, 51060-181-01, 51060-182-01, 51060-183-01, 51060-184-01, 51060-185-01, 51060-186-01, 51060-187-01, 51060-188-01, 51060-189-01, 51060-190-01, 51060-191-01, 51060-192-01, 51060-193-01, 51060-194-01, 51060-195-01, 51060-196-01 - Packager: Tarte, Inc
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 11, 2018
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
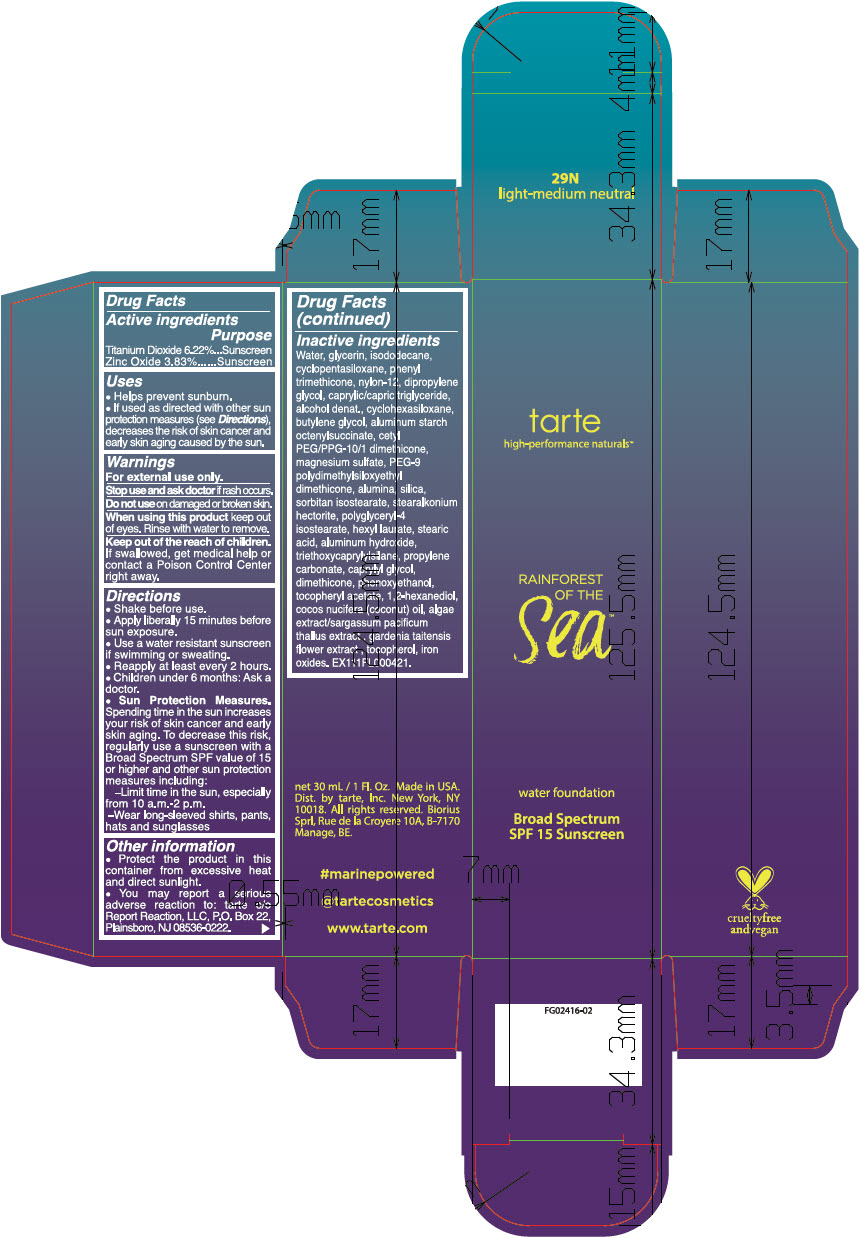
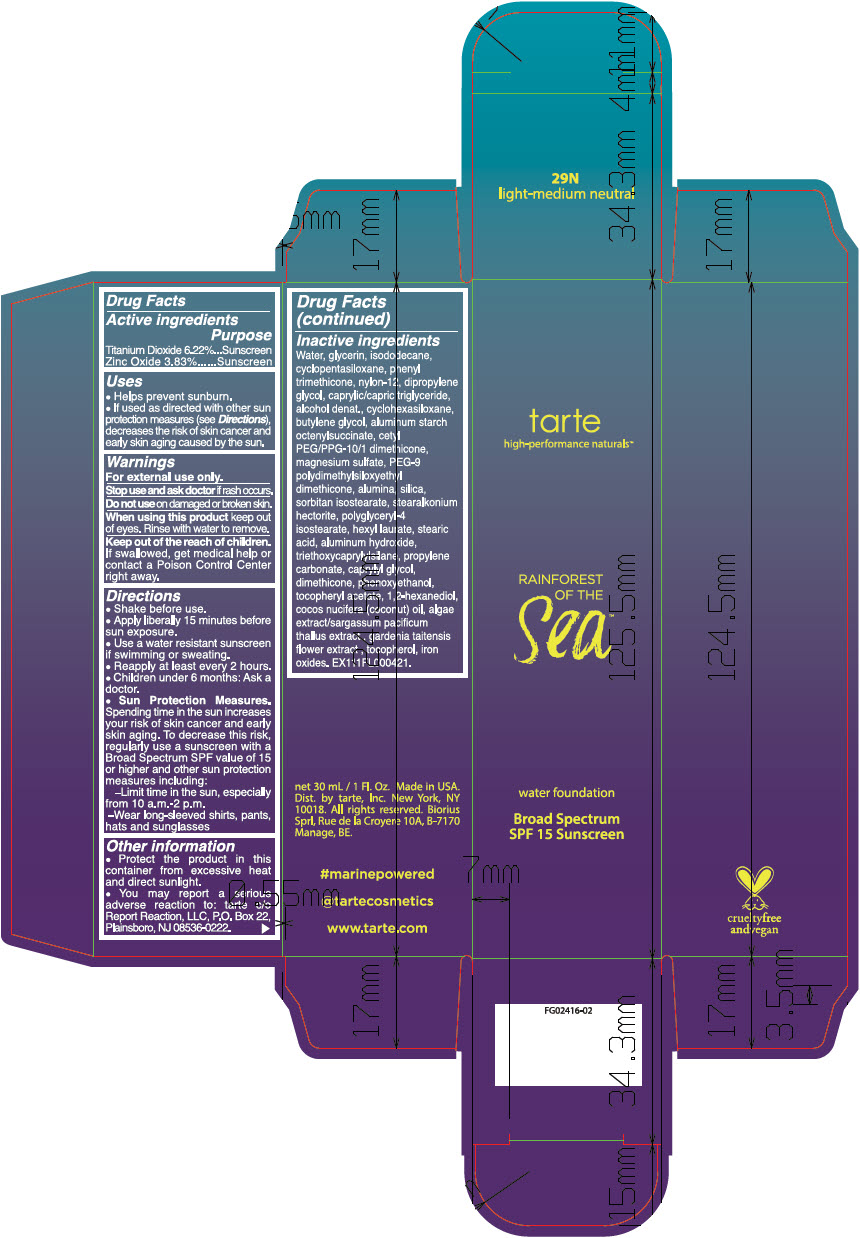
- ACTIVE INGREDIENT
-
Uses
- Helps prevent sunburn.
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- Warnings
-
Directions
- Shake before use.
- Apply liberally 15 minutes before sun exposure.
- Use a water resistant sunscreen if swimming or sweating.
- Reapply at least every 2 hours.
- Children under 6 months: Ask a doctor.
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- –
- Limit time in the sun, especially from 10 a.m.-2 p.m.
- –
- Wear long-sleeved shirts, pants, hats and sunglasses
- Other information
-
Inactive ingredients
Water, glycerin, isododecane, cyclopentasiloxane, phenyl trimethicone, nylon-12, dipropylene glycol, caprylic/capric triglyceride, alcohol denat., cyclohexasiloxane, butylene glycol, aluminum starch octenylsuccinate, cetyl PEG/PPG-10/1 dimethicone, magnesium sulfate, PEG-9 polydimethylsiloxyethyl dimethicone, alumina, silica, sorbitan isostearate, stearalkonium hectorite, polyglyceryl-4 isostearate, hexyl laurate, stearic acid, aluminum hydroxide, triethoxycaprylylsilane, propylene carbonate, caprylyl glycol, dimethicone, phenoxyethanol, tocopheryl acetate, 1,2-hexanediol, cocos nucifera (coconut) oil, algae extract/sargassum pacificum thallus extract , gardenia taitensis flower extract , tocopherol, iron oxides. EX111FL000421.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 8S porcelain
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 12B fair beige
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 12N fair neutral
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 14S fair-light sand
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 15N fair-light neutral
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 22N light neutral
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 22S light sand
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 22H light honey
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 22G light golden
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 27S light-medium sand
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 27B light-medium beige
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 27H light-medium honey
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 29N light-medium neutral
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 29G light-medium golden
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 32S medium sand
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 32H medium honey
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 32N medium neutral
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 32G medium golden
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 37S medium-tan sand
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 39G medium-tan golden
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 42N tan neutral
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 42S tan sand
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 46S tan-deep sand
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 48G tan-deep golden
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 51H deep honey
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 51S deep sand
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 53G deep golden
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 57N rich neutral
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 57S rich sand
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 61H mahogany
-
INGREDIENTS AND APPEARANCE
RAINFOREST OF THE SEA WATER FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 8S PORCELAIN
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-167 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 62.2 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 38.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ISODODECANE (UNII: A8289P68Y2) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) NYLON-12 (UNII: 446U8J075B) DIPROPYLENE GLYCOL (UNII: E107L85C40) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CYCLOMETHICONE 6 (UNII: XHK3U310BA) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) ALUMINUM OXIDE (UNII: LMI26O6933) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PROPYLENE CARBONATE (UNII: 8D08K3S51E) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) PHENOXYETHANOL (UNII: HIE492ZZ3T) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) COCONUT OIL (UNII: Q9L0O73W7L) GARDENIA TAITENSIS FLOWER (UNII: T0OCU8201Y) TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-167-01 1 in 1 CARTON 11/01/2018 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 RAINFOREST OF THE SEA WATER FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 12B FAIR BEIGE
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-168 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 62.2 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 38.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ISODODECANE (UNII: A8289P68Y2) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) NYLON-12 (UNII: 446U8J075B) DIPROPYLENE GLYCOL (UNII: E107L85C40) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CYCLOMETHICONE 6 (UNII: XHK3U310BA) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) ALUMINUM OXIDE (UNII: LMI26O6933) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PROPYLENE CARBONATE (UNII: 8D08K3S51E) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) PHENOXYETHANOL (UNII: HIE492ZZ3T) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) COCONUT OIL (UNII: Q9L0O73W7L) GARDENIA TAITENSIS FLOWER (UNII: T0OCU8201Y) TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-168-01 1 in 1 CARTON 11/01/2018 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 RAINFOREST OF THE SEA WATER FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 12N FAIR NEUTRAL
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-169 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 62.2 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 38.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ISODODECANE (UNII: A8289P68Y2) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) NYLON-12 (UNII: 446U8J075B) DIPROPYLENE GLYCOL (UNII: E107L85C40) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CYCLOMETHICONE 6 (UNII: XHK3U310BA) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) ALUMINUM OXIDE (UNII: LMI26O6933) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PROPYLENE CARBONATE (UNII: 8D08K3S51E) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) PHENOXYETHANOL (UNII: HIE492ZZ3T) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) COCONUT OIL (UNII: Q9L0O73W7L) GARDENIA TAITENSIS FLOWER (UNII: T0OCU8201Y) TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-169-01 1 in 1 CARTON 11/01/2018 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 RAINFOREST OF THE SEA WATER FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 14S FAIR-LIGHT SAND
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-170 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 62.2 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 38.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ISODODECANE (UNII: A8289P68Y2) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) NYLON-12 (UNII: 446U8J075B) DIPROPYLENE GLYCOL (UNII: E107L85C40) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CYCLOMETHICONE 6 (UNII: XHK3U310BA) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) ALUMINUM OXIDE (UNII: LMI26O6933) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PROPYLENE CARBONATE (UNII: 8D08K3S51E) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) PHENOXYETHANOL (UNII: HIE492ZZ3T) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) COCONUT OIL (UNII: Q9L0O73W7L) GARDENIA TAITENSIS FLOWER (UNII: T0OCU8201Y) TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-170-01 1 in 1 CARTON 11/01/2018 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 RAINFOREST OF THE SEA WATER FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 15N FAIR-LIGHT NEUTRAL
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-171 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 62.2 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 38.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ISODODECANE (UNII: A8289P68Y2) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) NYLON-12 (UNII: 446U8J075B) DIPROPYLENE GLYCOL (UNII: E107L85C40) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CYCLOMETHICONE 6 (UNII: XHK3U310BA) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) ALUMINUM OXIDE (UNII: LMI26O6933) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PROPYLENE CARBONATE (UNII: 8D08K3S51E) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) PHENOXYETHANOL (UNII: HIE492ZZ3T) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) COCONUT OIL (UNII: Q9L0O73W7L) GARDENIA TAITENSIS FLOWER (UNII: T0OCU8201Y) TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-171-01 1 in 1 CARTON 11/01/2018 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 RAINFOREST OF THE SEA WATER FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 22N LIGHT NEUTRAL
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-172 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 62.2 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 38.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ISODODECANE (UNII: A8289P68Y2) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) NYLON-12 (UNII: 446U8J075B) DIPROPYLENE GLYCOL (UNII: E107L85C40) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CYCLOMETHICONE 6 (UNII: XHK3U310BA) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) ALUMINUM OXIDE (UNII: LMI26O6933) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PROPYLENE CARBONATE (UNII: 8D08K3S51E) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) PHENOXYETHANOL (UNII: HIE492ZZ3T) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) COCONUT OIL (UNII: Q9L0O73W7L) GARDENIA TAITENSIS FLOWER (UNII: T0OCU8201Y) TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-172-01 1 in 1 CARTON 11/01/2018 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 RAINFOREST OF THE SEA WATER FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 22S LIGHT SAND
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-173 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 62.2 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 38.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ISODODECANE (UNII: A8289P68Y2) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) NYLON-12 (UNII: 446U8J075B) DIPROPYLENE GLYCOL (UNII: E107L85C40) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CYCLOMETHICONE 6 (UNII: XHK3U310BA) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) ALUMINUM OXIDE (UNII: LMI26O6933) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PROPYLENE CARBONATE (UNII: 8D08K3S51E) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) PHENOXYETHANOL (UNII: HIE492ZZ3T) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) COCONUT OIL (UNII: Q9L0O73W7L) GARDENIA TAITENSIS FLOWER (UNII: T0OCU8201Y) TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-173-01 1 in 1 CARTON 11/01/2018 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 RAINFOREST OF THE SEA WATER FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 22H LIGHT HONEY
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-174 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 62.2 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 38.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ISODODECANE (UNII: A8289P68Y2) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) NYLON-12 (UNII: 446U8J075B) DIPROPYLENE GLYCOL (UNII: E107L85C40) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CYCLOMETHICONE 6 (UNII: XHK3U310BA) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) ALUMINUM OXIDE (UNII: LMI26O6933) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PROPYLENE CARBONATE (UNII: 8D08K3S51E) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) PHENOXYETHANOL (UNII: HIE492ZZ3T) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) COCONUT OIL (UNII: Q9L0O73W7L) GARDENIA TAITENSIS FLOWER (UNII: T0OCU8201Y) TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-174-01 1 in 1 CARTON 11/01/2018 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 RAINFOREST OF THE SEA WATER FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 22G LIGHT GOLDEN
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-175 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 62.2 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 38.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ISODODECANE (UNII: A8289P68Y2) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) NYLON-12 (UNII: 446U8J075B) DIPROPYLENE GLYCOL (UNII: E107L85C40) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CYCLOMETHICONE 6 (UNII: XHK3U310BA) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) ALUMINUM OXIDE (UNII: LMI26O6933) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PROPYLENE CARBONATE (UNII: 8D08K3S51E) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) PHENOXYETHANOL (UNII: HIE492ZZ3T) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) COCONUT OIL (UNII: Q9L0O73W7L) GARDENIA TAITENSIS FLOWER (UNII: T0OCU8201Y) TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-175-01 1 in 1 CARTON 11/01/2018 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 RAINFOREST OF THE SEA WATER FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 27S LIGHT-MEDIUM SAND
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-176 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 62.2 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 38.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ISODODECANE (UNII: A8289P68Y2) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) NYLON-12 (UNII: 446U8J075B) DIPROPYLENE GLYCOL (UNII: E107L85C40) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CYCLOMETHICONE 6 (UNII: XHK3U310BA) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) ALUMINUM OXIDE (UNII: LMI26O6933) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PROPYLENE CARBONATE (UNII: 8D08K3S51E) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) PHENOXYETHANOL (UNII: HIE492ZZ3T) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) COCONUT OIL (UNII: Q9L0O73W7L) GARDENIA TAITENSIS FLOWER (UNII: T0OCU8201Y) TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-176-01 1 in 1 CARTON 11/01/2018 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 RAINFOREST OF THE SEA WATER FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 27B LIGHT-MEDIUM BEIGE
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-177 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 62.2 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 38.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ISODODECANE (UNII: A8289P68Y2) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) NYLON-12 (UNII: 446U8J075B) DIPROPYLENE GLYCOL (UNII: E107L85C40) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CYCLOMETHICONE 6 (UNII: XHK3U310BA) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) ALUMINUM OXIDE (UNII: LMI26O6933) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PROPYLENE CARBONATE (UNII: 8D08K3S51E) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) PHENOXYETHANOL (UNII: HIE492ZZ3T) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) COCONUT OIL (UNII: Q9L0O73W7L) GARDENIA TAITENSIS FLOWER (UNII: T0OCU8201Y) TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-177-01 1 in 1 CARTON 11/01/2018 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 RAINFOREST OF THE SEA WATER FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 27H LIGHT-MEDIUM HONEY
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-178 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 62.2 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 38.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ISODODECANE (UNII: A8289P68Y2) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) NYLON-12 (UNII: 446U8J075B) DIPROPYLENE GLYCOL (UNII: E107L85C40) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CYCLOMETHICONE 6 (UNII: XHK3U310BA) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) ALUMINUM OXIDE (UNII: LMI26O6933) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PROPYLENE CARBONATE (UNII: 8D08K3S51E) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) PHENOXYETHANOL (UNII: HIE492ZZ3T) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) COCONUT OIL (UNII: Q9L0O73W7L) GARDENIA TAITENSIS FLOWER (UNII: T0OCU8201Y) TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-178-01 1 in 1 CARTON 11/01/2018 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 RAINFOREST OF THE SEA WATER FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 29N LIGHT-MEDIUM NEUTRAL
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-179 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 62.2 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 38.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ISODODECANE (UNII: A8289P68Y2) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) NYLON-12 (UNII: 446U8J075B) DIPROPYLENE GLYCOL (UNII: E107L85C40) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CYCLOMETHICONE 6 (UNII: XHK3U310BA) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) ALUMINUM OXIDE (UNII: LMI26O6933) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PROPYLENE CARBONATE (UNII: 8D08K3S51E) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) PHENOXYETHANOL (UNII: HIE492ZZ3T) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) COCONUT OIL (UNII: Q9L0O73W7L) GARDENIA TAITENSIS FLOWER (UNII: T0OCU8201Y) TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-179-01 1 in 1 CARTON 11/01/2018 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 RAINFOREST OF THE SEA WATER FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 29G LIGHT-MEDIUM GOLDEN
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-180 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 62.2 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 38.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ISODODECANE (UNII: A8289P68Y2) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) NYLON-12 (UNII: 446U8J075B) DIPROPYLENE GLYCOL (UNII: E107L85C40) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CYCLOMETHICONE 6 (UNII: XHK3U310BA) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) ALUMINUM OXIDE (UNII: LMI26O6933) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PROPYLENE CARBONATE (UNII: 8D08K3S51E) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) PHENOXYETHANOL (UNII: HIE492ZZ3T) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) COCONUT OIL (UNII: Q9L0O73W7L) GARDENIA TAITENSIS FLOWER (UNII: T0OCU8201Y) TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-180-01 1 in 1 CARTON 11/01/2018 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 RAINFOREST OF THE SEA WATER FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 32S MEDIUM SAND
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-181 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 62.2 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 38.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ISODODECANE (UNII: A8289P68Y2) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) NYLON-12 (UNII: 446U8J075B) DIPROPYLENE GLYCOL (UNII: E107L85C40) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CYCLOMETHICONE 6 (UNII: XHK3U310BA) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) ALUMINUM OXIDE (UNII: LMI26O6933) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PROPYLENE CARBONATE (UNII: 8D08K3S51E) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) PHENOXYETHANOL (UNII: HIE492ZZ3T) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) COCONUT OIL (UNII: Q9L0O73W7L) GARDENIA TAITENSIS FLOWER (UNII: T0OCU8201Y) TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-181-01 1 in 1 CARTON 11/01/2018 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 RAINFOREST OF THE SEA WATER FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 32H MEDIUM HONEY
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-182 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 62.2 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 38.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ISODODECANE (UNII: A8289P68Y2) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) NYLON-12 (UNII: 446U8J075B) DIPROPYLENE GLYCOL (UNII: E107L85C40) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CYCLOMETHICONE 6 (UNII: XHK3U310BA) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) ALUMINUM OXIDE (UNII: LMI26O6933) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PROPYLENE CARBONATE (UNII: 8D08K3S51E) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) PHENOXYETHANOL (UNII: HIE492ZZ3T) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) COCONUT OIL (UNII: Q9L0O73W7L) GARDENIA TAITENSIS FLOWER (UNII: T0OCU8201Y) TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-182-01 1 in 1 CARTON 11/01/2018 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 RAINFOREST OF THE SEA WATER FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 32N MEDIUM NEUTRAL
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-183 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 62.2 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 38.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ISODODECANE (UNII: A8289P68Y2) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) NYLON-12 (UNII: 446U8J075B) DIPROPYLENE GLYCOL (UNII: E107L85C40) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CYCLOMETHICONE 6 (UNII: XHK3U310BA) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) ALUMINUM OXIDE (UNII: LMI26O6933) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PROPYLENE CARBONATE (UNII: 8D08K3S51E) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) PHENOXYETHANOL (UNII: HIE492ZZ3T) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) COCONUT OIL (UNII: Q9L0O73W7L) GARDENIA TAITENSIS FLOWER (UNII: T0OCU8201Y) TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-183-01 1 in 1 CARTON 11/01/2018 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 RAINFOREST OF THE SEA WATER FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 32G MEDIUM GOLDEN
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-184 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 62.2 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 38.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ISODODECANE (UNII: A8289P68Y2) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) NYLON-12 (UNII: 446U8J075B) DIPROPYLENE GLYCOL (UNII: E107L85C40) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CYCLOMETHICONE 6 (UNII: XHK3U310BA) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) ALUMINUM OXIDE (UNII: LMI26O6933) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PROPYLENE CARBONATE (UNII: 8D08K3S51E) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) PHENOXYETHANOL (UNII: HIE492ZZ3T) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) COCONUT OIL (UNII: Q9L0O73W7L) GARDENIA TAITENSIS FLOWER (UNII: T0OCU8201Y) TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-184-01 1 in 1 CARTON 11/01/2018 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 RAINFOREST OF THE SEA WATER FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 37S MEDIUM-TAN SAND
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-185 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 62.2 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 38.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ISODODECANE (UNII: A8289P68Y2) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) NYLON-12 (UNII: 446U8J075B) DIPROPYLENE GLYCOL (UNII: E107L85C40) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CYCLOMETHICONE 6 (UNII: XHK3U310BA) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) ALUMINUM OXIDE (UNII: LMI26O6933) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PROPYLENE CARBONATE (UNII: 8D08K3S51E) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) PHENOXYETHANOL (UNII: HIE492ZZ3T) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) COCONUT OIL (UNII: Q9L0O73W7L) GARDENIA TAITENSIS FLOWER (UNII: T0OCU8201Y) TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-185-01 1 in 1 CARTON 11/01/2018 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 RAINFOREST OF THE SEA WATER FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 39G MEDIUM-TAN GOLDEN
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-186 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 62.2 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 38.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ISODODECANE (UNII: A8289P68Y2) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) GLYCERIN (UNII: PDC6A3C0OX) NYLON-12 (UNII: 446U8J075B) CYCLOMETHICONE 6 (UNII: XHK3U310BA) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) DIPROPYLENE GLYCOL (UNII: E107L85C40) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM OXIDE (UNII: LMI26O6933) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) HEXYL LAURATE (UNII: 4CG9F9W01Q) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) CAPRYLYL GLYCOL (UNII: 00YIU5438U) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) PROPYLENE CARBONATE (UNII: 8D08K3S51E) COCONUT OIL (UNII: Q9L0O73W7L) GARDENIA TAITENSIS FLOWER (UNII: T0OCU8201Y) TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-186-01 1 in 1 CARTON 11/01/2018 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 RAINFOREST OF THE SEA WATER FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 42N TAN NEUTRAL
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-187 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 62.2 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 38.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ISODODECANE (UNII: A8289P68Y2) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) NYLON-12 (UNII: 446U8J075B) DIPROPYLENE GLYCOL (UNII: E107L85C40) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CYCLOMETHICONE 6 (UNII: XHK3U310BA) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) ALUMINUM OXIDE (UNII: LMI26O6933) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PROPYLENE CARBONATE (UNII: 8D08K3S51E) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) PHENOXYETHANOL (UNII: HIE492ZZ3T) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) COCONUT OIL (UNII: Q9L0O73W7L) GARDENIA TAITENSIS FLOWER (UNII: T0OCU8201Y) TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-187-01 1 in 1 CARTON 11/01/2018 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 RAINFOREST OF THE SEA WATER FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 42S TAN SAND
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-188 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 62.2 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 38.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ISODODECANE (UNII: A8289P68Y2) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) NYLON-12 (UNII: 446U8J075B) DIPROPYLENE GLYCOL (UNII: E107L85C40) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CYCLOMETHICONE 6 (UNII: XHK3U310BA) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) ALUMINUM OXIDE (UNII: LMI26O6933) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PROPYLENE CARBONATE (UNII: 8D08K3S51E) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) PHENOXYETHANOL (UNII: HIE492ZZ3T) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) COCONUT OIL (UNII: Q9L0O73W7L) GARDENIA TAITENSIS FLOWER (UNII: T0OCU8201Y) TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-188-01 1 in 1 CARTON 11/01/2018 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 RAINFOREST OF THE SEA WATER FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 46S TAN-DEEP SAND
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-189 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 62.2 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 38.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ISODODECANE (UNII: A8289P68Y2) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) NYLON-12 (UNII: 446U8J075B) DIPROPYLENE GLYCOL (UNII: E107L85C40) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CYCLOMETHICONE 6 (UNII: XHK3U310BA) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) ALUMINUM OXIDE (UNII: LMI26O6933) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PROPYLENE CARBONATE (UNII: 8D08K3S51E) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) PHENOXYETHANOL (UNII: HIE492ZZ3T) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) COCONUT OIL (UNII: Q9L0O73W7L) GARDENIA TAITENSIS FLOWER (UNII: T0OCU8201Y) TOCOPHEROL (UNII: R0ZB2556P8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-189-01 1 in 1 CARTON 11/01/2018 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 RAINFOREST OF THE SEA WATER FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 48G TAN-DEEP GOLDEN
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-190 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 62.2 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 38.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ISODODECANE (UNII: A8289P68Y2) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) GLYCERIN (UNII: PDC6A3C0OX) NYLON-12 (UNII: 446U8J075B) CYCLOMETHICONE 6 (UNII: XHK3U310BA) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) DIPROPYLENE GLYCOL (UNII: E107L85C40) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM OXIDE (UNII: LMI26O6933) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) HEXYL LAURATE (UNII: 4CG9F9W01Q) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) CAPRYLYL GLYCOL (UNII: 00YIU5438U) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) PROPYLENE CARBONATE (UNII: 8D08K3S51E) COCONUT OIL (UNII: Q9L0O73W7L) GARDENIA TAITENSIS FLOWER (UNII: T0OCU8201Y) TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-190-01 1 in 1 CARTON 11/01/2018 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 RAINFOREST OF THE SEA WATER FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 51H DEEP HONEY
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-191 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 62.2 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 38.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ISODODECANE (UNII: A8289P68Y2) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) GLYCERIN (UNII: PDC6A3C0OX) NYLON-12 (UNII: 446U8J075B) CYCLOMETHICONE 6 (UNII: XHK3U310BA) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) DIPROPYLENE GLYCOL (UNII: E107L85C40) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM OXIDE (UNII: LMI26O6933) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) HEXYL LAURATE (UNII: 4CG9F9W01Q) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) CAPRYLYL GLYCOL (UNII: 00YIU5438U) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) PROPYLENE CARBONATE (UNII: 8D08K3S51E) COCONUT OIL (UNII: Q9L0O73W7L) GARDENIA TAITENSIS FLOWER (UNII: T0OCU8201Y) TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-191-01 1 in 1 CARTON 11/01/2018 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 RAINFOREST OF THE SEA WATER FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 51S DEEP SAND
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-192 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 62.2 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 38.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ISODODECANE (UNII: A8289P68Y2) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) GLYCERIN (UNII: PDC6A3C0OX) NYLON-12 (UNII: 446U8J075B) CYCLOMETHICONE 6 (UNII: XHK3U310BA) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) DIPROPYLENE GLYCOL (UNII: E107L85C40) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM OXIDE (UNII: LMI26O6933) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) HEXYL LAURATE (UNII: 4CG9F9W01Q) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) CAPRYLYL GLYCOL (UNII: 00YIU5438U) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) PROPYLENE CARBONATE (UNII: 8D08K3S51E) COCONUT OIL (UNII: Q9L0O73W7L) GARDENIA TAITENSIS FLOWER (UNII: T0OCU8201Y) TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-192-01 1 in 1 CARTON 11/01/2018 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 RAINFOREST OF THE SEA WATER FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 53G DEEP GOLDEN
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-193 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 62.2 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 38.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ISODODECANE (UNII: A8289P68Y2) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) GLYCERIN (UNII: PDC6A3C0OX) NYLON-12 (UNII: 446U8J075B) CYCLOMETHICONE 6 (UNII: XHK3U310BA) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) DIPROPYLENE GLYCOL (UNII: E107L85C40) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM OXIDE (UNII: LMI26O6933) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) HEXYL LAURATE (UNII: 4CG9F9W01Q) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) CAPRYLYL GLYCOL (UNII: 00YIU5438U) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) PROPYLENE CARBONATE (UNII: 8D08K3S51E) COCONUT OIL (UNII: Q9L0O73W7L) GARDENIA TAITENSIS FLOWER (UNII: T0OCU8201Y) TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-193-01 1 in 1 CARTON 11/01/2018 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 RAINFOREST OF THE SEA WATER FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 57N RICH NEUTRAL
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-194 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 62.2 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 38.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ISODODECANE (UNII: A8289P68Y2) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) GLYCERIN (UNII: PDC6A3C0OX) NYLON-12 (UNII: 446U8J075B) CYCLOMETHICONE 6 (UNII: XHK3U310BA) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) DIPROPYLENE GLYCOL (UNII: E107L85C40) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM OXIDE (UNII: LMI26O6933) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) HEXYL LAURATE (UNII: 4CG9F9W01Q) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) CAPRYLYL GLYCOL (UNII: 00YIU5438U) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) PROPYLENE CARBONATE (UNII: 8D08K3S51E) COCONUT OIL (UNII: Q9L0O73W7L) GARDENIA TAITENSIS FLOWER (UNII: T0OCU8201Y) TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-194-01 1 in 1 CARTON 11/01/2018 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 RAINFOREST OF THE SEA WATER FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 57S RICH SAND
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-195 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 62.2 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 38.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ISODODECANE (UNII: A8289P68Y2) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) GLYCERIN (UNII: PDC6A3C0OX) NYLON-12 (UNII: 446U8J075B) CYCLOMETHICONE 6 (UNII: XHK3U310BA) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) DIPROPYLENE GLYCOL (UNII: E107L85C40) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM OXIDE (UNII: LMI26O6933) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) HEXYL LAURATE (UNII: 4CG9F9W01Q) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) CAPRYLYL GLYCOL (UNII: 00YIU5438U) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) PROPYLENE CARBONATE (UNII: 8D08K3S51E) COCONUT OIL (UNII: Q9L0O73W7L) GARDENIA TAITENSIS FLOWER (UNII: T0OCU8201Y) TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-195-01 1 in 1 CARTON 11/01/2018 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 RAINFOREST OF THE SEA WATER FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 61H MAHOGANY
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-196 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 62.2 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 38.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ISODODECANE (UNII: A8289P68Y2) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) GLYCERIN (UNII: PDC6A3C0OX) NYLON-12 (UNII: 446U8J075B) CYCLOMETHICONE 6 (UNII: XHK3U310BA) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) DIPROPYLENE GLYCOL (UNII: E107L85C40) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM OXIDE (UNII: LMI26O6933) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) HEXYL LAURATE (UNII: 4CG9F9W01Q) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) CAPRYLYL GLYCOL (UNII: 00YIU5438U) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) PROPYLENE CARBONATE (UNII: 8D08K3S51E) COCONUT OIL (UNII: Q9L0O73W7L) GARDENIA TAITENSIS FLOWER (UNII: T0OCU8201Y) TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-196-01 1 in 1 CARTON 11/01/2018 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 Labeler - Tarte, Inc (027905186)