PROACTIV CONCEALER PLUS- sulfur lotion
THE PROACTIV COMPANY LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Proactiv Solution Concealer Plus
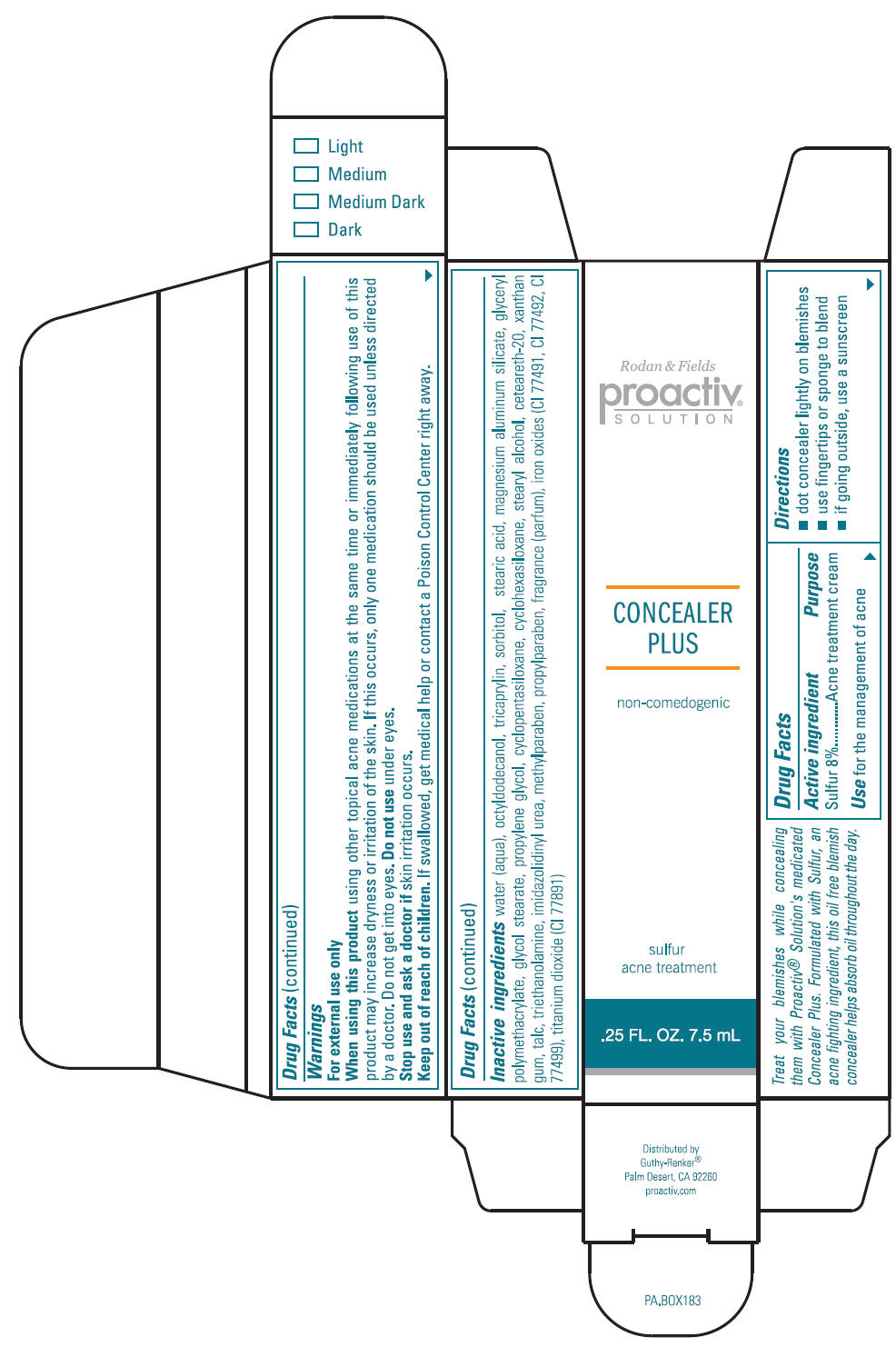
Directions
- dot concealer lightly on blemishes
- use fingertips or sponge to blend
- if going outside, use a sunscreen
Warnings
For external use only
Inactive Ingredients
water (aqua), octyldodecanol, tricaprylin, sorbitol, stearic acid, magnesium aluminum silicate, glyceryl polymethacrylate, glycol stearate, propylene glycol, cyclopentasiloxane, cyclohexasiloxane, stearyl alcohol, ceteareth-20, xanthan gum, talc, triethanolamine, imidazolidinyl urea, methylparaben, propylparaben, fragrance (parfum), iron oxides (CI 77491, CI 77492, CI 77499), titanium dioxide (CI 77891)
| PROACTIV
CONCEALER PLUS
sulfur lotion |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - THE PROACTIV COMPANY LLC (080216357) |
| Registrant - THE PROACTIV COMPANY LLC (080216357) |
Revised: 1/2020
Document Id: a75d8386-0825-4fe8-87bb-8a0f6bbce7dc
Set id: 6c0e1bd5-59ee-4770-a7c0-47435697d400
Version: 2
Effective Time: 20200108
THE PROACTIV COMPANY LLC