DIPHENHYDRAMINE HCL 25 MG- diphenhydramine hcl tablet
Medline Industries, LP
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
990 Diphenhydramine HCL 25mg
Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- itchy, watery eyes
- sneezing
- itchy nose or throat
temporarily relieves these symptoms due to the common cold:
- runny nose
- sneezing
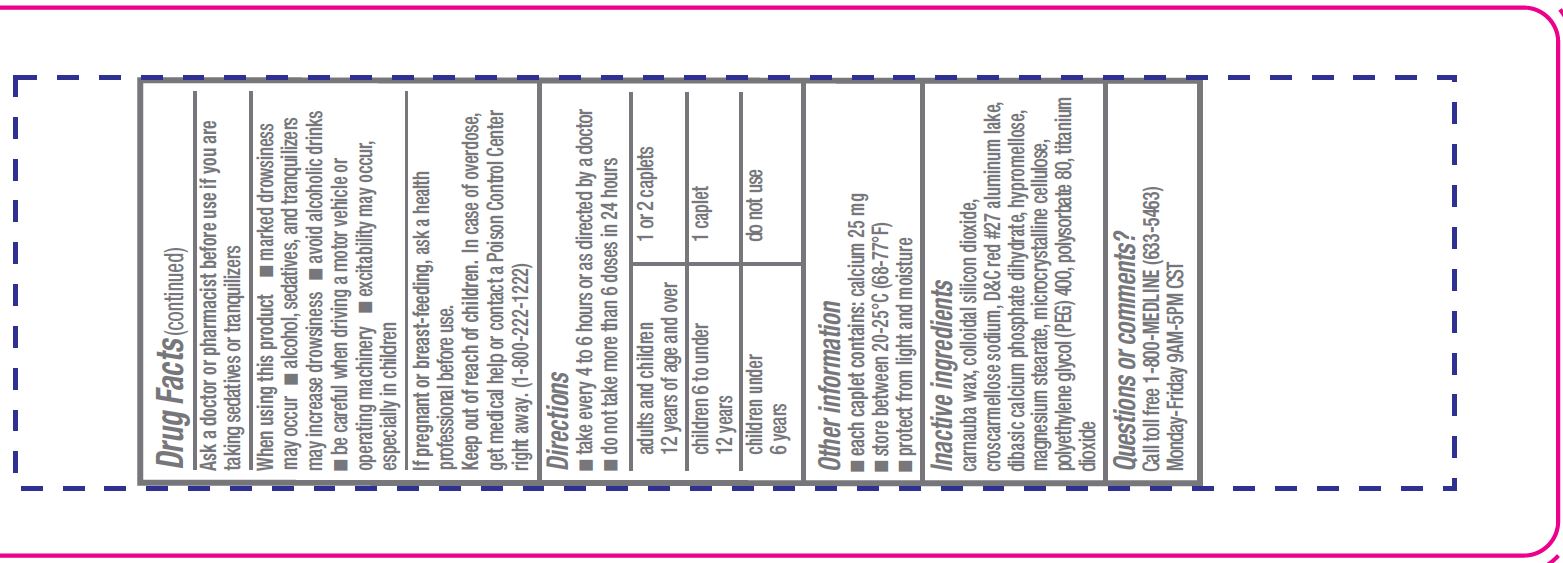
Warnings
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
Directions
- take every 4 to 6 hours or as directed by a doctor
- do not take more than 6 doses in 24 hours
adults and children 12 years of age and over 1 or 2 caplets children 6 to under 12 years 1 caplet children under 6 years do not use this product for children under 6 years of age
- do not take more than 6 doses in 24 hours
Other information
- each caplet contains: calcium 25 mg
- store between 20°-25°C (68°-77°F)
- protect from light and moisture
| DIPHENHYDRAMINE HCL 25 MG
diphenhydramine hcl tablet |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Medline Industries, LP (025460908) |
| Registrant - Medline Industries, LP (025460908) |
Revised: 10/2022
Document Id: ea26da62-3255-4f1a-e053-2a95a90a3400
Set id: 6bde8f0a-a388-77a7-e053-2a91aa0a04b4
Version: 5
Effective Time: 20221003
Medline Industries, LP