SENNA- sennosides syrup
GERI-CARE PHARMACEUTICAL CORP
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
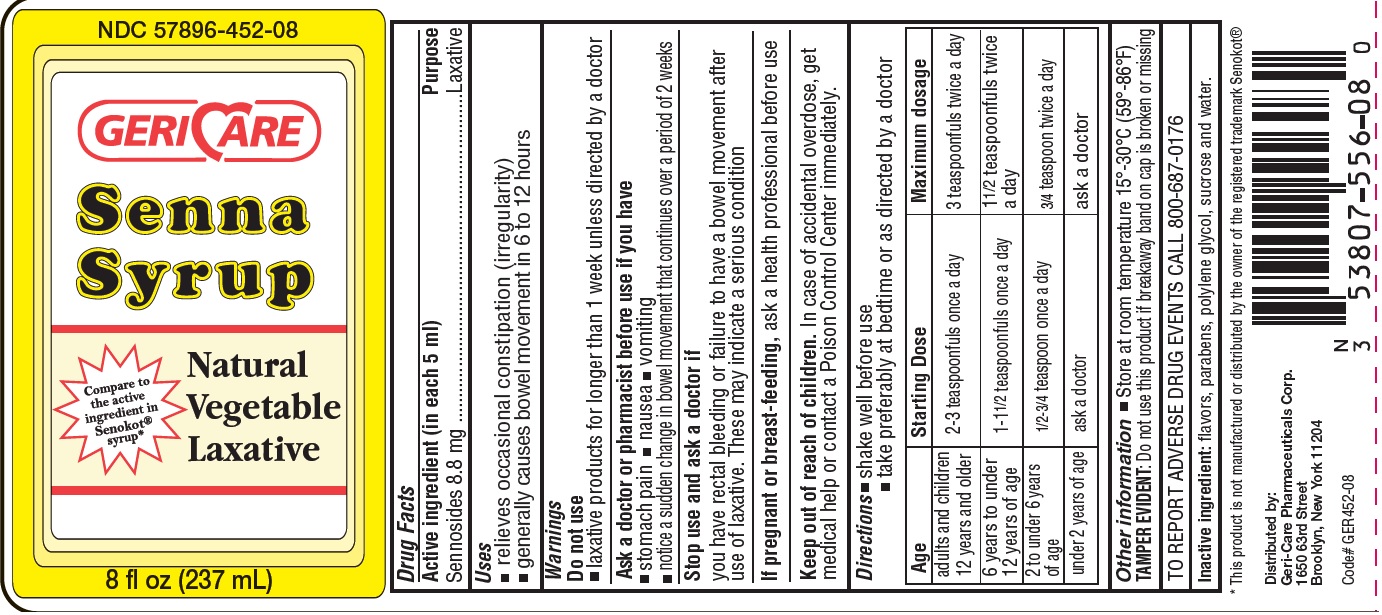
GC Senna Syrup
Warnings
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel movements that continues over a period of 2 weeks
Directions
- shake well before use
- take preferably at bedtime or as directed by a doctor
| Age | Starting Dose | Maximum dosage |
|---|---|---|
| adults and children 12 years and older | 2 - 3 teaspoons once a day | 3 teaspoons twice a day |
| 6 years to under
12 years of age | 1-11/2 teaspoonfuls once a day | 11/2 teaspoonfuls twice a day |
| 2 to under 6 years
of age | 1/2-3/4 teaspoon once a day | 3/4 teaspoon twice a day |
| Under 2 years of age | ask a doctor | ask a doctor |
| SENNA
sennosides syrup |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - GERI-CARE PHARMACEUTICAL CORP (611196254) |
| Registrant - GERI-CARE PHARMACEUTICAL CORP (611196254) |
Revised: 4/2021
Document Id: bff0be20-03f9-ab5a-e053-2a95a90aff01
Set id: 6bca97d4-e389-6808-e053-2991aa0a98e3
Version: 3
Effective Time: 20210414
GERI-CARE PHARMACEUTICAL CORP