Label: NIZORAL PSORIASIS- salicylic acid shampoo
- NDC Code(s): 55505-202-64
- Packager: Kramer Laboratories
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 13, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INDICATIONS & USAGE
- WARNINGS
- Ask a doctor before use if
- When using this product
- Stop use and ask a doctor if
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
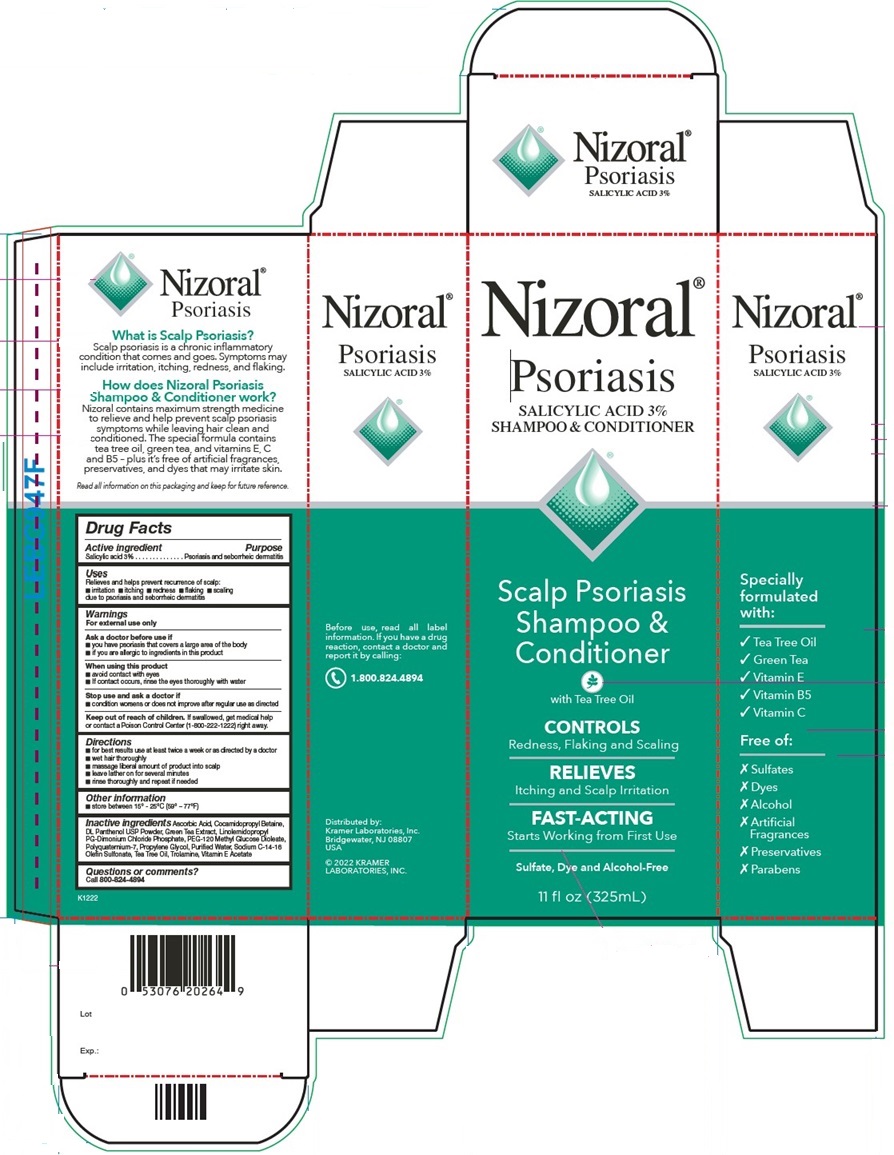
Principal Display Panel
Nizoral®
Psoriasis
SALICYLIC ACID 3%
SHAMPOO & CONDITIONER
Scalp Psoriasis
Shampoo &
Conditioner
with Tea Tree Oil
CONTROLS
Redness, Flaking and Scaling
RELIEVES
Itching and Scalp Irritation
FAST-ACTING
Starts Working from First Use
Sulfate, Dye and Alcohol-Free
11 fl oz (325mL)
Specially formulated with:
✓ Tea Tree Oil
✓ Green Tea
✓ Vitamin E
✓ Vitamin B5
✓ Vitamin C
Free of:
X Sulfates
X Dyes
X Alcohol
X Artificial Fragrances
X Preservatives
X Parabens
What is Scalp Psoriasis?
Scalp psoriasis is a chronic inflammatory
condition that comes and goes. Symptoms may
include irritation, itching, redness, and flaking.
How does Nizoral Psoriasis
Shampoo & Conditioner work?
Nizoral contains maximum strength medicine
to relieve and help prevent scalp psoriasis
symptoms while leaving hair clean and
conditioned. The special formula contains
tea tree oil, green tea and vitamins E, C
and B5 - plus it's free of artificial fragrances,
preservatives, and dyes that may irritate skin.
Read all information on this packaging and keep for future reference.
Before use, read all label
information. If you have a drug
reaction, contact a doctor and
report it by calling:
1.800.824.4894
Distributed by:
Kramer Laboratories, Inc.
Bridgewater, NJ 08807
USA
© 2022 KRAMER
LABORATORIES, INC.
K1222
Nizoral®
Psoriasis
SALICYLIC ACID 3%
SHAMPOO & CONDITIONER
Scalp Psoriasis
Shampoo &
Conditioner
with Tea Tree Oil
CONTROLS
Redness, Flaking and Scaling
RELIEVES
Itching and Scalp Irritation
FAST-ACTING
Starts Working from First Use
Sulfate, Dye and Alcohol-Free
11 fl oz (325mL)

-
INGREDIENTS AND APPEARANCE
NIZORAL PSORIASIS
salicylic acid shampooProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55505-202 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Salicylic Acid (UNII: O414PZ4LPZ) (Salicylic Acid - UNII:O414PZ4LPZ) Salicylic Acid 3 g in 100 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Sodium C14-16 Olefin Sulfonate (UNII: O9W3D3YF5U) Cocamidopropyl Betaine (UNII: 5OCF3O11KX) Peg-120 Methyl Glucose Dioleate (UNII: YM0K64F20V) Polyquaternium-7 (70/30 Acrylamide/Dadmac; 1600000 Mw) (UNII: 0L414VCS5Y) Trolamine (UNII: 9O3K93S3TK) Panthenol (UNII: WV9CM0O67Z) .Alpha.-Tocopherol Acetate (UNII: 9E8X80D2L0) Tea Tree Oil (UNII: VIF565UC2G) Green Tea Leaf (UNII: W2ZU1RY8B0) Ascorbic Acid (UNII: PQ6CK8PD0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55505-202-64 1 in 1 CARTON 12/01/2020 1 325 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M032 12/01/2020 Labeler - Kramer Laboratories (122720675)