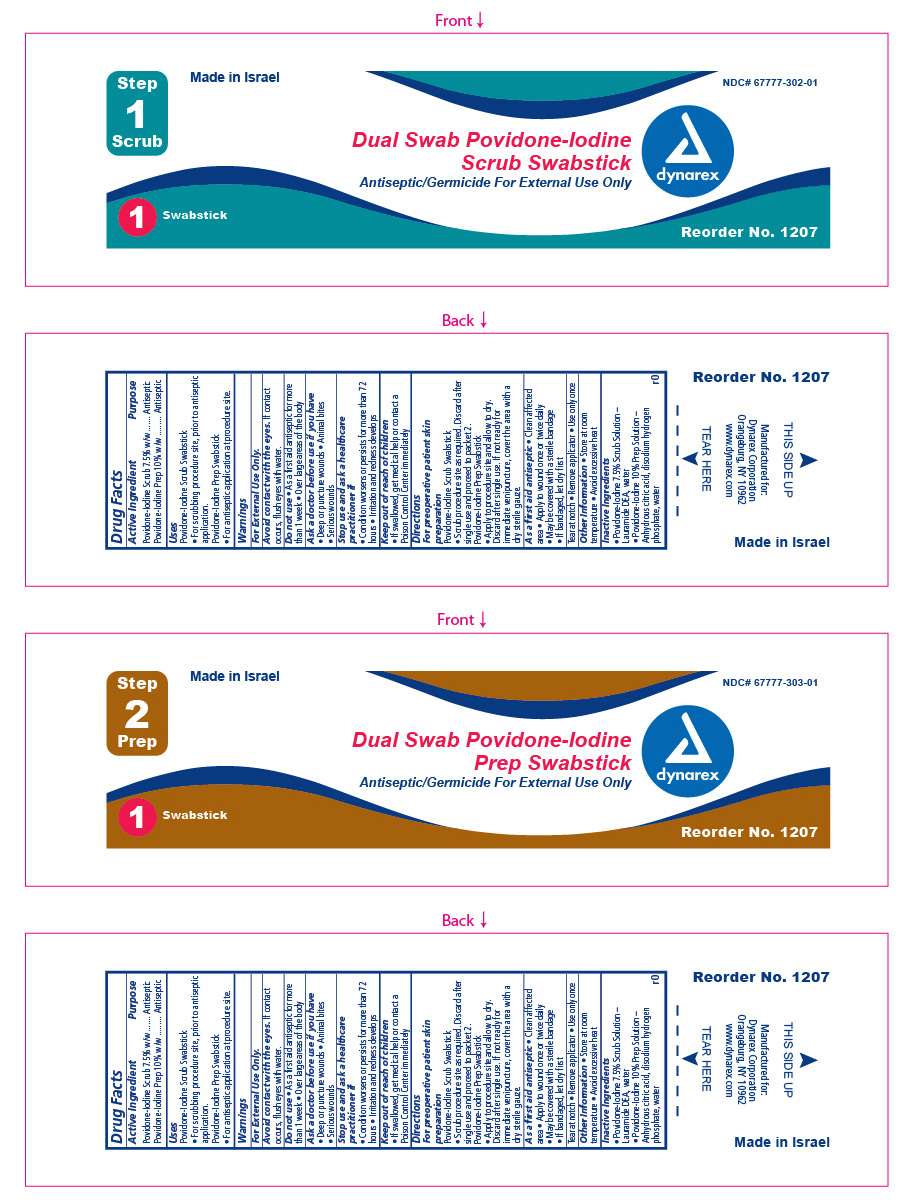
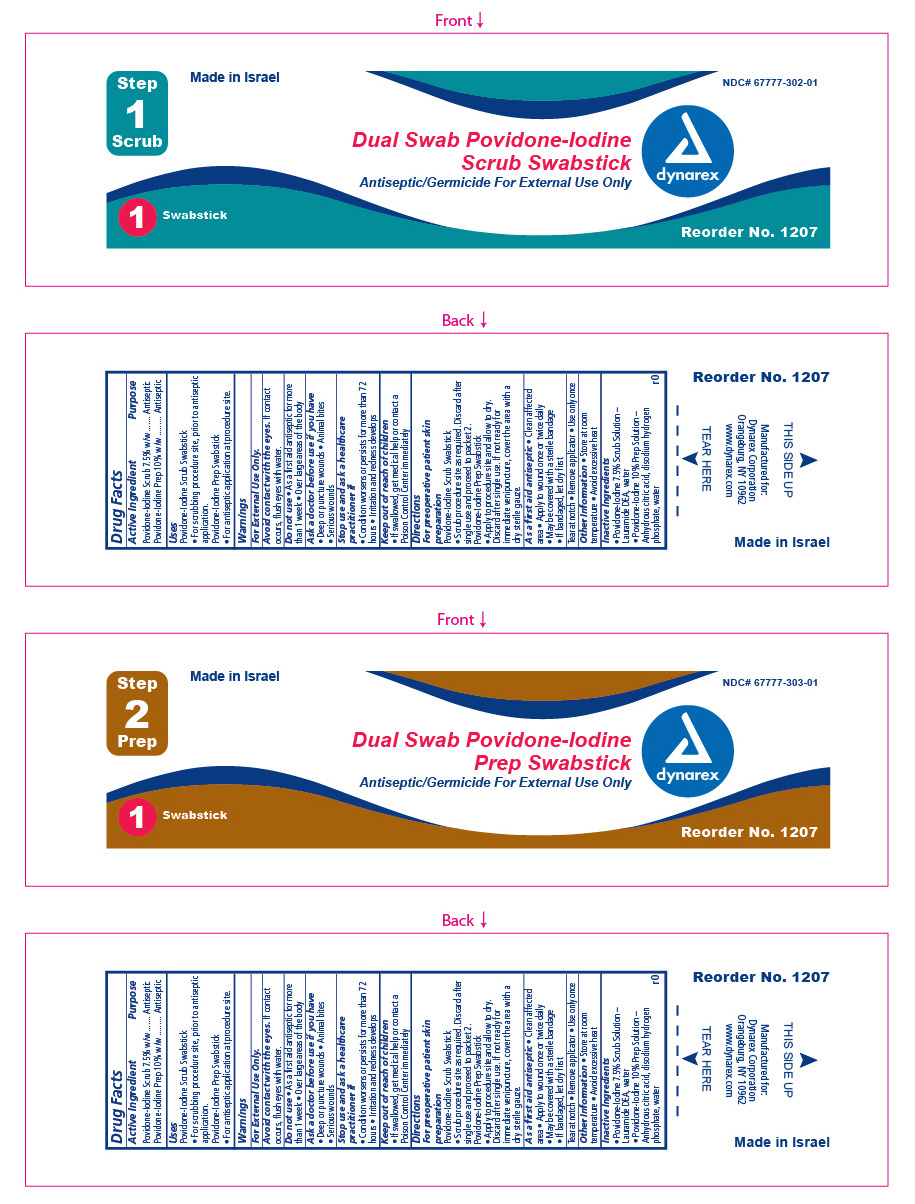
Label: DUAL SWAB POVIDONE-IODINE PREP- povidone iodine kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 67777-302-01, 67777-303-01, 67777-414-01 - Packager: Dynarex Corporation
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 24, 2020
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- Purpose
- SPL UNCLASSIFIED SECTION
- INDICATIONS & USAGE
- Warnings
- Stop Use
- Ask a Doctor
- DO NOT USE
- KEEP OUT OF REACH OF CHILDREN
-
Dosage and Administration
Directions
For pre-operative patient skin preparation:
- Apply to procedure site and allow to dry.
- discard after single use.
- If not ready for immediate venipuncture, cover the area with a dry sterile gauze.
As a first aid antiseptic:
- clean affected area
- apply to wound once or twice daily
- may be covered with a sterile bandage
- if bandaged let dry first
Tear at notch, remove applicator, use only once.
- Inactive Ingredients
- Other information
- Principal Display panel
-
INGREDIENTS AND APPEARANCE
DUAL SWAB POVIDONE-IODINE PREP
povidone iodine kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67777-414 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67777-414-01 1 in 1 PACKAGE; Type 1: Convenience Kit of Co-Package 04/05/2017 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 25 POUCH 32.5 mL Part 2 25 POUCH 32.5 mL Part 1 of 2 DUAL SWAB POVIDONE-IODINE PREP
povidone iodine swabProduct Information Item Code (Source) NDC:67777-302 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 7.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength LAURIC DIETHANOLAMIDE (UNII: I29I2VHG38) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67777-302-01 1.3 mL in 1 POUCH; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333C Part 2 of 2 DUAL SWAB POVIDONE-IODINE PREP
povidone iodine swabProduct Information Item Code (Source) NDC:67777-303 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) SODIUM PHOSPHATE, DIBASIC DODECAHYDRATE (UNII: E1W4N241FO) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67777-303-01 1.3 mL in 1 POUCH; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333C Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333C 11/15/2015 Labeler - Dynarex Corporation (008124539) Registrant - Dynarex Corporation (008124539)