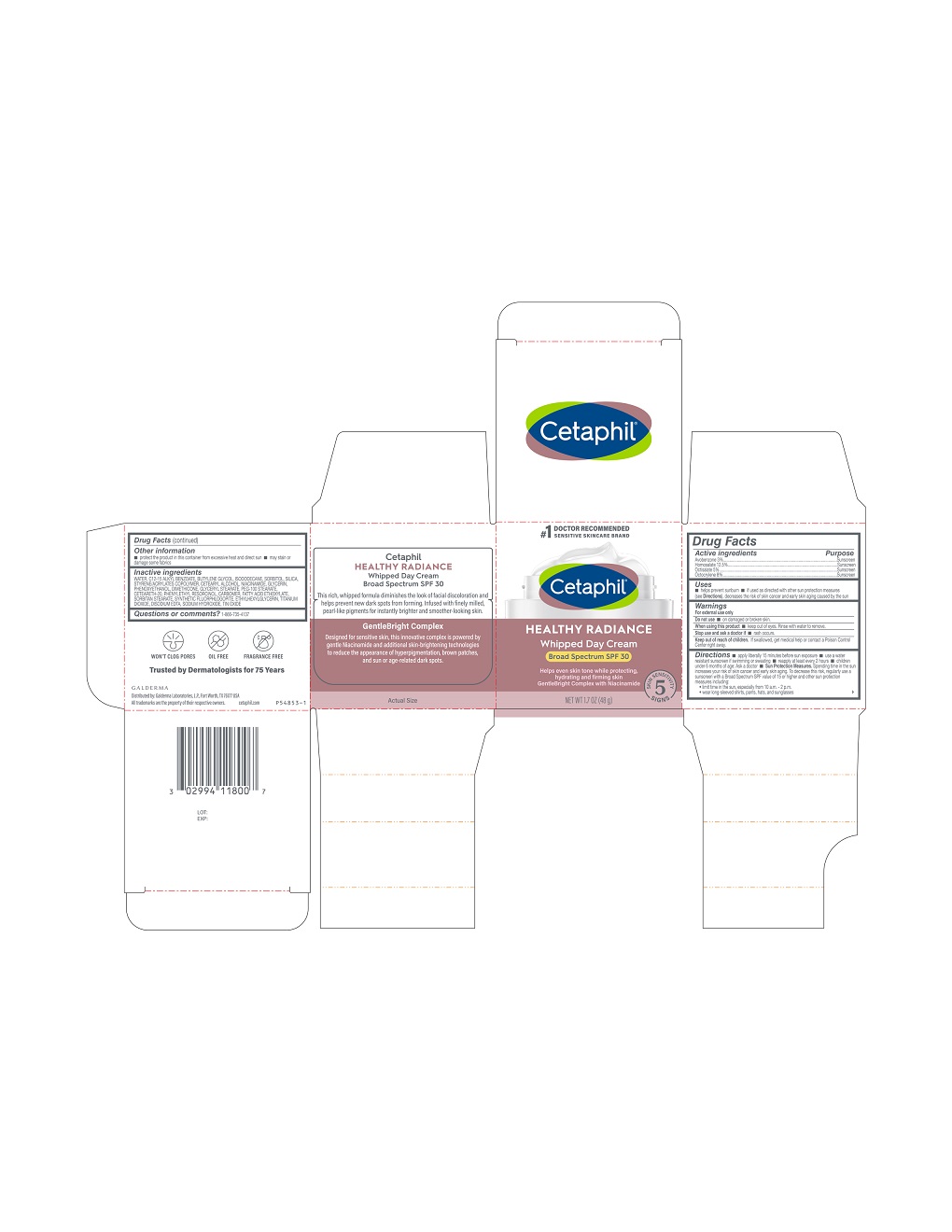
Label: CETAPHIL HEALTHY RADIANCE WHIPPED DAY CREAM BROAD SPECTRUM SPF 30- avobenzone, homosalate, octisalate, octocrylene lotion
- NDC Code(s): 0299-4118-00
- Packager: Galderma Laboratories, L.P.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated November 28, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients.....Purpose
- OTC - PURPOSE SECTION
- Purpose
- Uses
- Warnings
- Do not use
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
-
Directions
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
- children under 6 months of age: Ask a doctor
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
-
Inactive Ingredients
Water, C12-15 Alkyl Benzoate, Butylene Glycol, Isododecane, Sorbitol, Silica, Styrene/Acrylates Copolymer, Cetearyl Alcohol, Niacinamide, Glycerin, Phenoxyethanol, Dimethicone, Glyceryl Stearate, PEG-100 Stearate, Ceteareth-20, Phenylethyl Resorcinol, Carbomer, Fatty Acid Ethoxylate, Sorbitan Stearate, Synthetic Fluorphlogopite, Ethylhexylglycerin, Titanium Dioxide, Disodium EDTA, Sodium Hydroxide, Tin Oxide
- Questions or comments?
-
Principal Display Panel - 3 FL OZ Carton
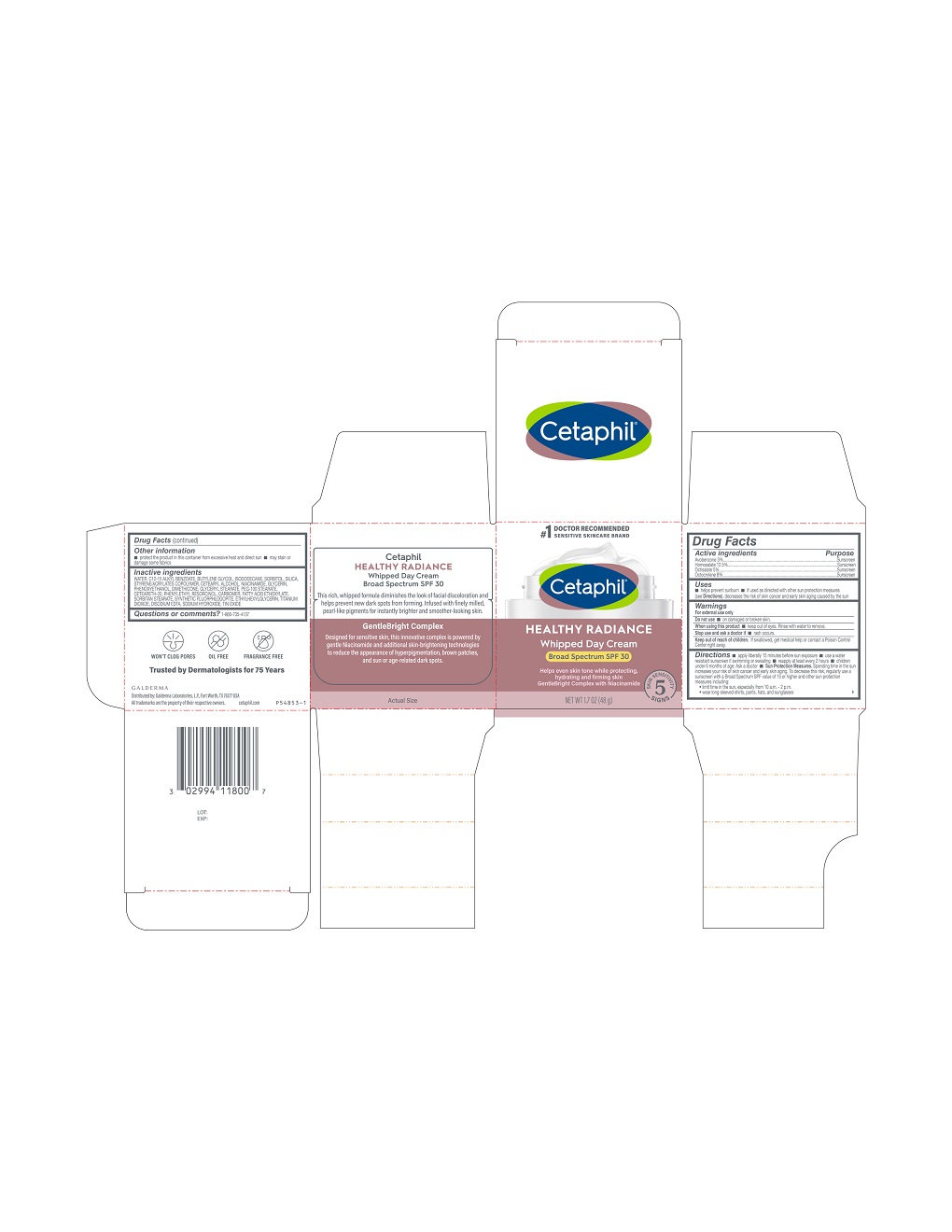
#1 Doctor Recommended
Sensitive Skincare Brand
Cetaphil®
HEALTHY RADIANCEWhipped Day Cream
Broad Spectrum SPF 30
Helps even skin tone while protecting,
hydrating and firming skin
GentleBright Complex with Niacinamide
Five Skin Sensitivity Signs
NET WT 1.7 OZ (48 g)
Distributed by:
Galderma Laboratories, L.P., Fort Worth, TX 76177 USA
All trademarks are the property of their respective owners.
Cetaphil.com
P54853-1
-
INGREDIENTS AND APPEARANCE
CETAPHIL HEALTHY RADIANCE WHIPPED DAY CREAM BROAD SPECTRUM SPF 30
avobenzone, homosalate, octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0299-4118 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Avobenzone (UNII: G63QQF2NOX) (Avobenzone - UNII:G63QQF2NOX) Avobenzone 30 mg in 1 g Homosalate (UNII: V06SV4M95S) (Homosalate - UNII:V06SV4M95S) Homosalate 125 mg in 1 g Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 50 mg in 1 g Octocrylene (UNII: 5A68WGF6WM) (Octocrylene - UNII:5A68WGF6WM) Octocrylene 80 mg in 1 g Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Alkyl (C12-15) Benzoate (UNII: A9EJ3J61HQ) Butylene Glycol (UNII: 3XUS85K0RA) Isododecane (UNII: A8289P68Y2) Sorbitol (UNII: 506T60A25R) Silicon Dioxide (UNII: ETJ7Z6XBU4) Styrene/Acrylamide Copolymer (500000 Mw) (UNII: 5Z4DPO246A) Cetostearyl Alcohol (UNII: 2DMT128M1S) Niacinamide (UNII: 25X51I8RD4) Glycerin (UNII: PDC6A3C0OX) Phenoxyethanol (UNII: HIE492ZZ3T) Dimethicone (UNII: 92RU3N3Y1O) Glyceryl Monostearate (UNII: 230OU9XXE4) Peg-100 Stearate (UNII: YD01N1999R) Polyoxyl 20 Cetostearyl Ether (UNII: YRC528SWUY) Phenylethyl Resorcinol (UNII: G37UFG162O) Carbomer Homopolymer, Unspecified Type (UNII: 0A5MM307FC) Sorbitan Monostearate (UNII: NVZ4I0H58X) Magnesium Potassium Aluminosilicate Fluoride (UNII: YK3DC63Y5M) Ethylhexylglycerin (UNII: 147D247K3P) Titanium Dioxide (UNII: 15FIX9V2JP) Edetate Disodium Anhydrous (UNII: 8NLQ36F6MM) Sodium Hydroxide (UNII: 55X04QC32I) Stannic Oxide (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0299-4118-00 1 in 1 CARTON 05/01/2021 1 48 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/01/2021 Labeler - Galderma Laboratories, L.P. (047350186) Establishment Name Address ID/FEI Business Operations Cosmetic Essence, LLC dba Voyant Beauty 032565959 manufacture(0299-4118)