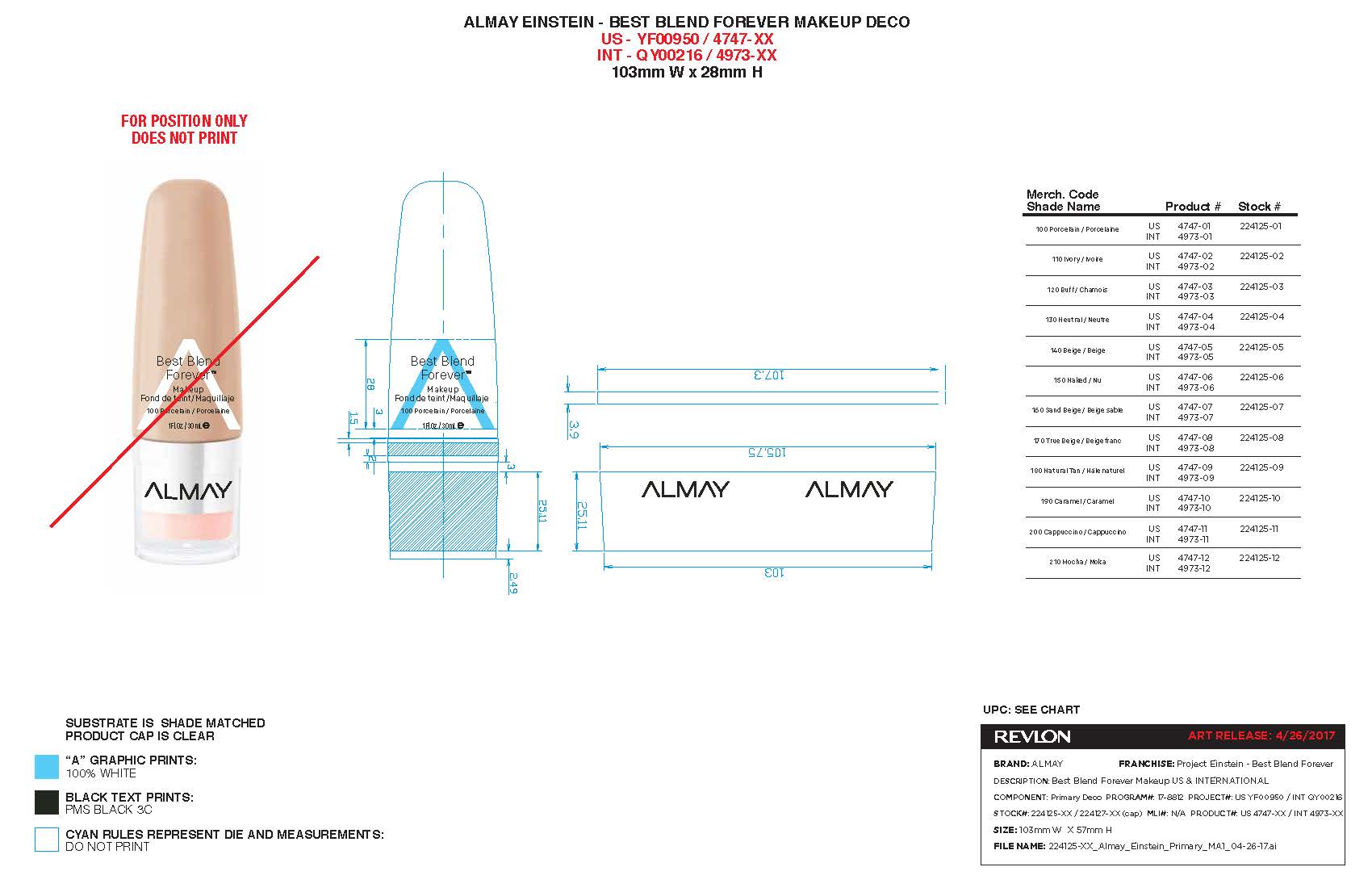
ALMAY BEST BLEND FOREVER MAKEUP- homosalate, octinoxate, octisalate, titanium dioxide lotion
Almay, Inc.
----------
Almay Best Blend Forever Makeup
Active Ingredients
HOMOSALATE 3.5%
OCTINOXATE 7.5g%
OCTISALATE 5.0g%
TITANIUM DIOXIDE 5.3%
Purpose:
Sunscreen
Warnings
For external use only
Do not us on damaged or broken skin
When using this product: Keep out of the eyes. Rinse with water to remove.
Stop use and ask a doctor if rash occurs.
Keep out of reach of children. If product is swallowed, get medical help or contact a Poison Control center right away.
Directions:
Apply liberaly 15 minutes before sun exposure.
Use a water resistant sunscreen if swimming or sweating.
Reapply at least every 2 hours.
Children under 6 months: Ask a doctor.
Sun Protection Measures:
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease the risk, reguraly use sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
Limit time in the sun, especially from 10am - 2pm
Wear long-sleeved shirts, pants, hats and sunglasses.
Inactive Ingredients
AQUA/WATER/EAU,BUTYLENE GLYCOL, GLYCERIN, POTASSIUM CETYL PHOSPHATE, CETEARYL ALCOHOL, DIMETHICONE, STEARETH-2, STEARETH-21, VP/EICOSENE COPOLYMER, CETYL ESTERS, ISOCETYL STEARATE, TRIDECYL TRIMELLITATE, PHENYL TRIMETHICONE, SILICA, ASCORBYL GLUCOSIDE, DIPOTASSIUM GLYCYRRHIZATE, GLYCYRRHIZA GLABRA (LICORICE) ROOT EXTRACT, HYDROLYZED GLYCOSAMINOGLYCANS, MARIS SAL ((SEA SALT) SEL MARIN), MORUS BOMBYCIS ROOT EXTRACT, NIACINAMIDE, POLYQUATERNIUM-51, PROPYLENE GLYCOL, SAXIFRAGA SARMENTOSA EXTRACT, SCUTELLARIA BAICALENSIS ROOT EXTRACT, SODIUM CARRAGEENAN, SODIUM HYALURONATE, SODIUM PCA, TREHALOSE, UREA, VITIS VINIFERA (GRAPE) FRUIT EXTRACT, ALUMINA, ETHYLENE BRASSYLATE, HEXYLENE GLYCOL, HYDROGEN DIMETHICONE, LECITHIN, MAGNESIUM ALUMINUM SILICATE, POLYSORBATE 20, PROPYLENE GLYCOL LAURATE, PROPYLENE GLYCOL STEARATE, SIMETHICONE, SORBITAN LAURATE, STEARIC ACID, TETRASODIUM EDTA, TRIACETIN, XANTHAN GUM, 1,2-HEXANEDIOL, CAPRYLYL GLYCOL, PHENOXYETHANOL,
May Contain: IRON OXIDES (CI 77491, CI 77492, CI 77499), MICA, TITANIUM DIOXIDE (CI 77891)
Uses
Helps Prevent Sunburn.
If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
| ALMAY BEST BLEND FOREVER MAKEUP
homosalate, octinoxate, octisalate, titanium dioxide lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Almay, Inc. (064988652) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| REVLON, INC | 809725570 | manufacture(0311-0719) | |