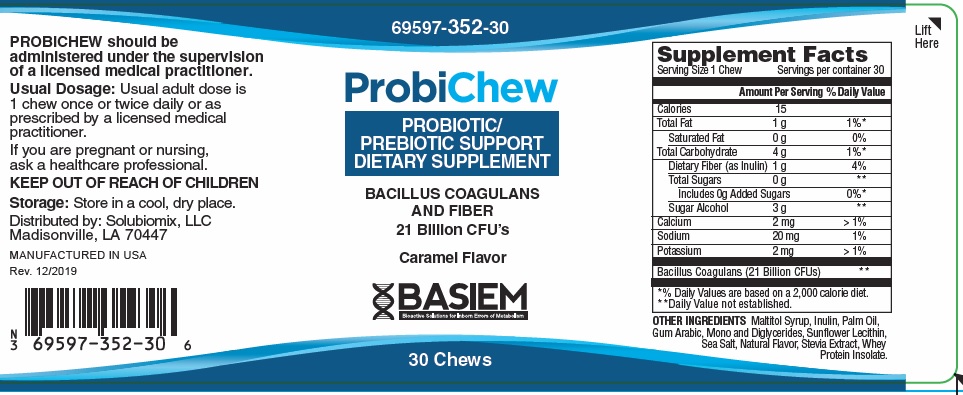
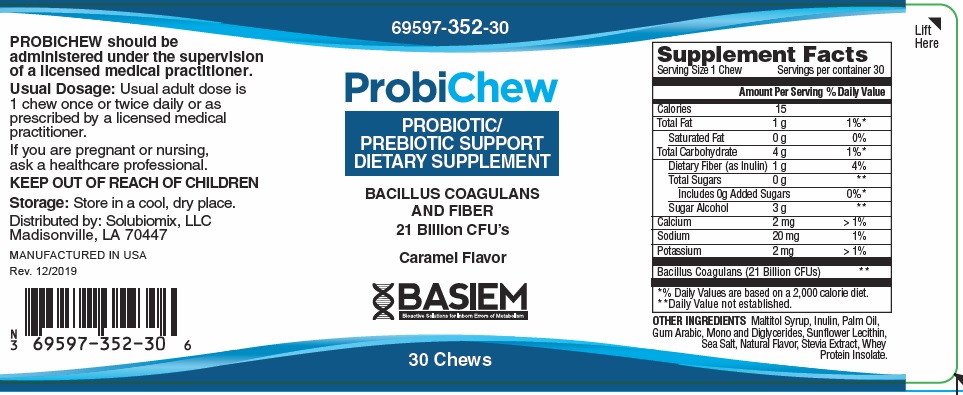
Label: PROBICHEW PROBIOTIC SUPPORT- bacillus coagulans and fiber bar, chewable
- NHRIC Code(s): 69597-352-30
- Packager: Basiem, LLC
- Category: DIETARY SUPPLEMENT
Drug Label Information
Updated October 3, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
STATEMENT OF IDENTITY
PROBICHEW Dietary Supplemement
Dispensed by Prescription
Amount Per Serving: %DV
Bacillus Coagulans21 Billion * Dietary Fiber (as Inulin) 1 gram 4% *Daily Values (DV) not established 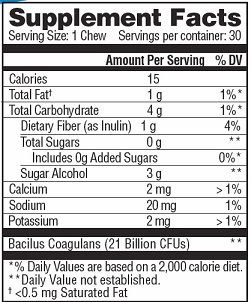
OTHER INGREDIENTS: Sodium, Potassium, Calcium, Sugar Alcohol, Maltitol Syrup, Inulin, Palm Oil, Gum Arabic, Mono and Diglycerides, Sunflower Lecithin, Sea Salt, Natural Flavor, Stevia Extract, Whey Protein Isolate
PROBICHEW is an orally administered prescription probiotic/prebiotic formulation for the clinical dietary management of suboptimal nutritional status in patients where advanced supplementation is required and nutritional supplementation in physiologically stressful conditions for maintenance of good health is needed.
PROBICHEW should be administered under the supervision of a licensed medical practitioner.
PROBICHEW is manufactured in accordance with Current Good Manufacturing Practice for foods, using ingredients that have been approved by the U.S. Food and Drug Administration (FDA) as food additives or are Generally Recognized as Safe (GRAS) for their intended use
-
DOSAGE
Usual adult dose is 1 chew once or twice daily, or as prescribed by a licensed medical practitioner.
If you are pregnant or nursing, ask a healthcare professional.
PROBICHEW should be administered under the supervision of a licensed medical practitioner.
DIRECTIONS FOR USE: REMOVE FOIL BEFORE CONSUMING CHEW. Consume entire chew or as directed by a healthcare professional.
-
SAFE HANDLING
This product is contraindicated in patients with a known hypersensitivity to any of the ingredients.
PROBICHEW chews should only be used under the direction and supervision of a licensed medical practitioner. Use with caution in patients that may have a medical condition, are pregnant, lactating, trying to conceive, under the age of 18, or taking medications.
KEEP OUT OF REACH OF CHILDREN
- STORAGE
-
HOW SUPPLIED HEALTH CLAIM
PROBICHEW, Probiotic/Prebiotic Support
Dietary Supplement
Chews are beige in color and individually wrapped in a bronze and silver foil.
Bottles contain 30 Chews 69597-350-30*
Manufactured in USA for:
Basiem, LLCMadisonville, LA 70447
Rev. 12/2019
*Basiem does not represent these product codes to be National Drug Codes (NDC). Product codes are formatted according to standard industry practice, to meet the formatting requirement by pedigree reporting and supply-chain control including pharmacies.
† The most appropriate way to ensure pedigree reporting consistent with these regulatory guidelines and safety monitoring is to dispense this product by prescription. This is not an Orange Book product. This product may be administered only under a physician’s supervision and all prescriptions using this product shall be pursuant to state statutes as applicable. The ingredients, indication or claims of this product are not to be construed to be drug claims.
- PROBICHEW LABEL
-
INGREDIENTS AND APPEARANCE
PROBICHEW PROBIOTIC SUPPORT
bacillus coagulans and fiber bar, chewableProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:69597-352 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACILLUS COAGULANS (UNII: ISK1LOY57E) (BACILLUS COAGULANS - UNII:ISK1LOY57E) BACILLUS COAGULANS 21000000000 [CFU] INULIN (UNII: JOS53KRJ01) (INULIN - UNII:JOS53KRJ01) INULIN 1000 mg Inactive Ingredients Ingredient Name Strength MALTITOL (UNII: D65DG142WK) PALM OIL (UNII: 5QUO05548Z) ACACIA (UNII: 5C5403N26O) LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) SEA SALT (UNII: 87GE52P74G) STEVIA REBAUDIUNA LEAF (UNII: 6TC6NN0876) WHEY PROTEIN HYDROLYSATE (UNII: 237DZG2JLA) SODIUM (UNII: 9NEZ333N27) POTASSIUM (UNII: RWP5GA015D) CALCIUM (UNII: SY7Q814VUP) MANNITOL (UNII: 3OWL53L36A) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:69597-352-30 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT 12/06/2019 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value scoring 1 shape size (solid drugs) 28 mm flavor color Labeler - Basiem, LLC (079686680)