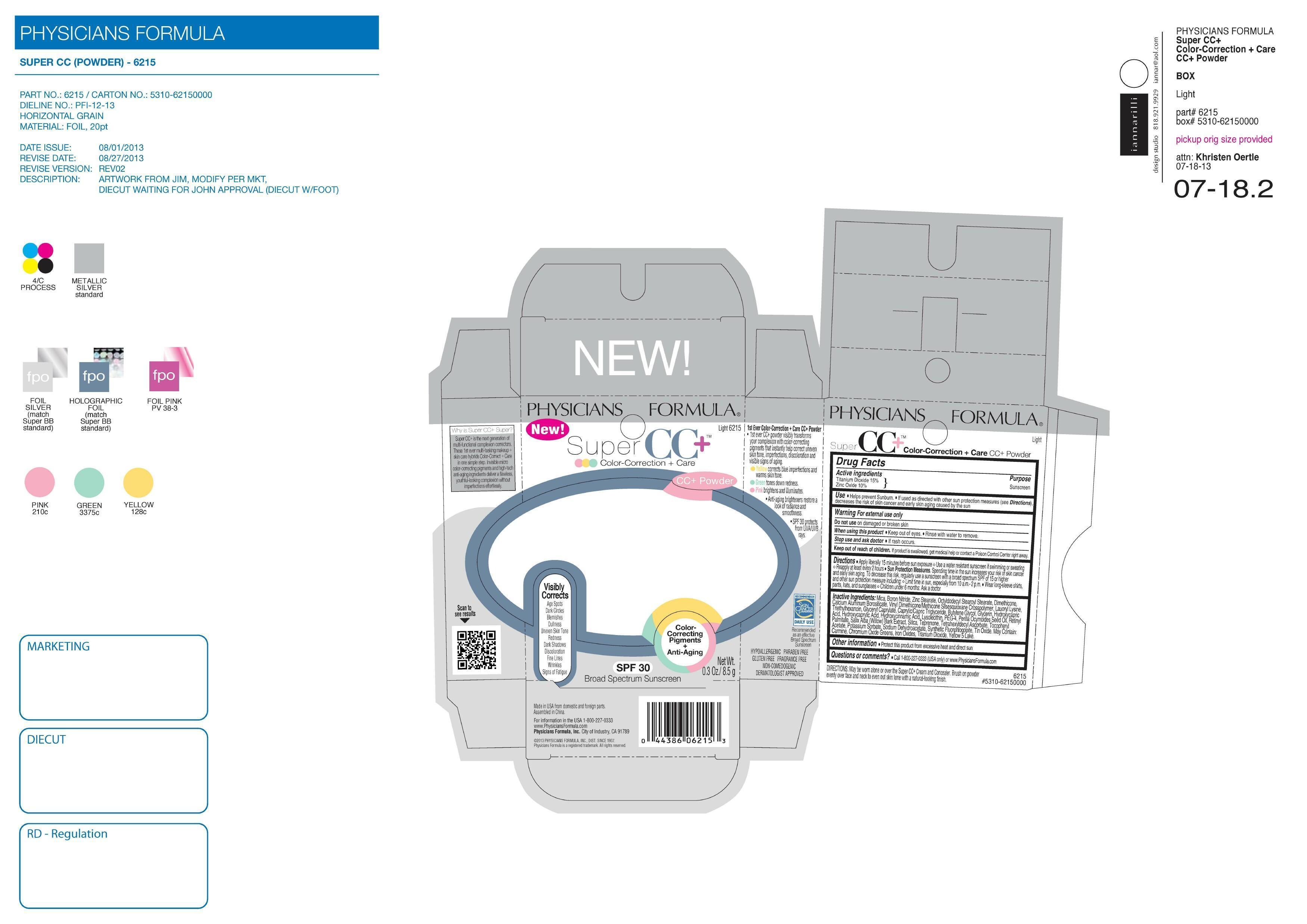
SUPER CC PLUS POWDER SPF 30- titanium dioxide, zinc oxide powder
Physicians Formula
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Super CC Powder-PF-Cosm Grp
| SUPER CC PLUS POWDER
SPF 30
titanium dioxide, zinc oxide powder |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Physicians Formula (021261805) |
| Registrant - Physicians Formula (021261805) |
Revised: 12/2019
Document Id: 9a04509d-2973-fd96-e053-2995a90ad178
Set id: 67528eb1-a3bd-40ab-8375-ffad07f941ca
Version: 3
Effective Time: 20191218
Physicians Formula