Label: WORM AND PARASITES AQUARIUM CURE PROGRAM FRESH- praziquantel kit
- NDC Code(s): 86048-001-01, 86048-002-01, 86048-011-01
- Packager: Prodibio SAS
- Category: OTC ANIMAL DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated August 27, 2015
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- INSTRUCTIONS
- PRESENTATION
- SPL UNCLASSIFIED SECTION
- - ACTIVE INGREDIENTS
- - EXCIPIENTS
- - PHARMACEUTICAL FORM & CONTENTS
- - MANUFACTURER
-
USAGE
- THERAPEUTIC INDICATIONS
Product intended only for treatment of ornamental freshwater fish infested with skin worms (Gyrodactylus), gill worms (Dactylogyrus), or tapeworms (Cestodes). Visible symptoms are fish rubbing against the scenery (to get rid of the parasites) in the case of Gyrodactylus; increased respiration for Dactylogyrus; and weight loss despite normal food intake for Cestodes. -
- DOSAGE AND DIRECTIONS FOR USE
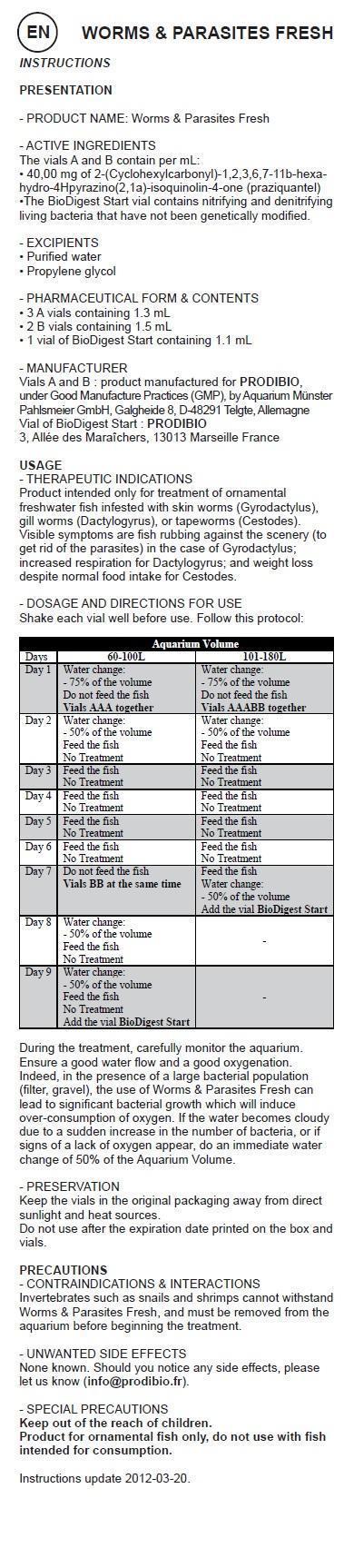
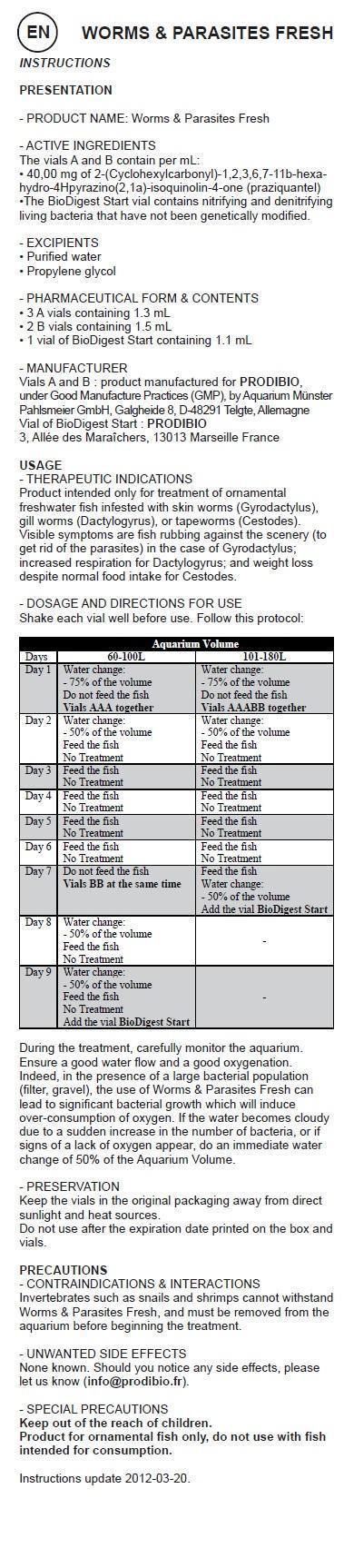
Shake each vial well before use. Follow this protocol:
Aquarium Volume Days 60-100L 101-180L Day 1 Water change:
- 75% of the volume
Do not feed the fish
Vials AAA togetherWater change:
- 75% of the volume
Do not feed the fish
Vials AAABB togetherDay 2 Water change:
- 50% of the volume
Feed the fish
No TreatmentWater change:
- 50% of the volume
Feed the fish
No TreatmentDay 3 Feed the fish
No TreatmentFeed the fish
No TreatmentDay 4 Feed the fish
No TreatmentFeed the fish
No TreatmentDay 5 Feed the fish
No TreatmentFeed the fish
No TreatmentDay 6 Feed the fish
No TreatmentFeed the fish
No TreatmentDay 7 Do not feed the fish
Vials BB at the same timeFeed the fish
Water change:
- 50% of the volume
Add the vial BioDigest StartDay 8 Water change:
- 50% of the volume
Feed the fish
No TreatmentDay 9 Water change:
- 50% of the volume
Feed the fish
No Treatment
Add the vial BioDigest StartDuring the treatment, carefully monitor the aquarium. Ensure a good water flow and a good oxygenation. Indeed, in the presence of a large bacterial population (filter, gravel), the use of Worms & Parasites Fresh can lead to significant bacterial growth which will induce over-consumption of oxygen. If the water becomes cloudy due to a sudden increase in the number of bacteria, or if signs of a lack of oxygen appear, do an immediate water change of 50% of the Aquarium Volume.
- - PRESERVATION
- PRECAUTIONS- CONTRAINDICATIONS & INTERACTIONS
- WARNINGS AND PRECAUTIONS
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
WORM AND PARASITES AQUARIUM CURE PROGRAM FRESH
praziquantel kitProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:86048-001 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:86048-001-01 1 in 1 BOX Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 3 VIAL, SINGLE-USE 3.9 mL Part 2 2 VIAL, SINGLE-USE 3 mL Part 1 of 2 A VIAL
praziquantel solutionProduct Information Item Code (Source) NDC:86048-002 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PRAZIQUANTEL (UNII: 6490C9U457) (PRAZIQUANTEL - UNII:6490C9U457) PRAZIQUANTEL 40 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:86048-002-01 1.3 mL in 1 VIAL, SINGLE-USE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 08/24/2015 Part 2 of 2 B VIAL
praziquantel solutionProduct Information Item Code (Source) NDC:86048-011 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PRAZIQUANTEL (UNII: 6490C9U457) (PRAZIQUANTEL - UNII:6490C9U457) PRAZIQUANTEL 40 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:86048-011-01 1.5 mL in 1 VIAL, SINGLE-USE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 08/24/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 08/24/2015 Labeler - Prodibio SAS (647909431)