DIPHENHYDRAMINE HCL- diphenhydramine hcl tablet
Rugby Laboratories Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
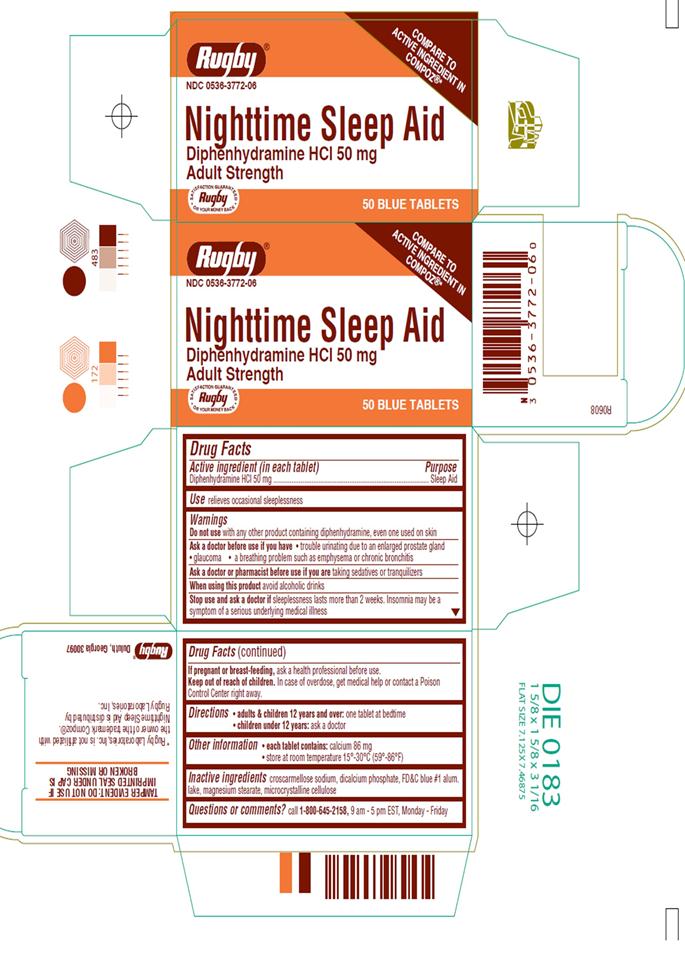
Nighttime Sleep Aid
Warnings
Do not use with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- •
- trouble urinating due to an enlarged prostate gland
- •
- glaucoma
- •
- a breathing problem such as emphysema or chronic bronchitis
Ask a doctor or pharmacist before use if you are taking sedatives or tranquilizers
When using this product
- •
- avoid alcoholic drinks
- Stop and ask a doctor if sleeplessness lasts more than 2 weeks. Insomnia may be a symptom of a serious underlying medical illness
- If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Direction
- •
- adults & children 12 years and over: one tablet at bedtime
- •
- children under 12 years: ask a doctor
Other Information
- •
- each tablet contains: calcium 86 mg
- •
- store at room temperature 15°-30°C (59°-86°F)
Inactive Ingredient
croscarmellose sodium, dicalcium phosphate, FD&C blue #1 alum.lake, magnesium stearate, microcrystalline cellulose
| DIPHENHYDRAMINE HCL
diphenhydramine hcl tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Rugby Laboratories Inc. (079246066) |
Revised: 12/2019
Document Id: 09dfadd2-8a5f-4127-aa06-fe727f418b53
Set id: 66d3cb86-df2c-443f-9bdd-79995b55d0a4
Version: 4
Effective Time: 20191220
Rugby Laboratories Inc.