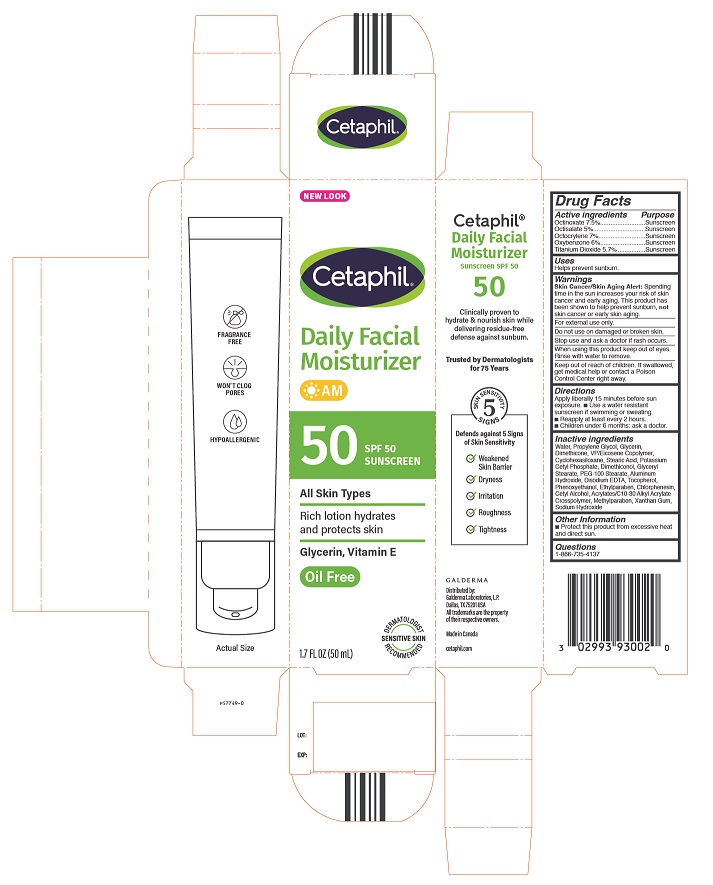
Label: CETAPHIL DAILY FACIAL MOISTURIZER WITH SUNCREEN SPF 50- octinoxate, octisalate, octocrylene, oxybenzone, titanium dioxide lotion
- NDC Code(s): 0299-4930-02
- Packager: Galderma Laboratories, L.P.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
- Warnings
- Directions
-
Inactive Ingredients
Water, Propylene Glycol, Glycerin, Dimethicone, VP/Eicosene Copolymer ,Cyclohexasiloxane, Stearic Acid, Potassium Cetyl Phosphate, Dimethiconol, Glyceryl Stearate, PEG-100 Stearate, Aluminum Hydroxide, Disodium EDTA, Tocopherol, Phenoxyethanol, Ethylparaben, Chlorphenesin, Cetyl Alcohol, Acrylates/C10-30 Alkyl Acrylate, Crosspolymer, Methylparaben, Xanthan Gum, Sodium Hydroxide
- Other Information
- Questions?
-
Principal Display Panel - 1.7oz carton
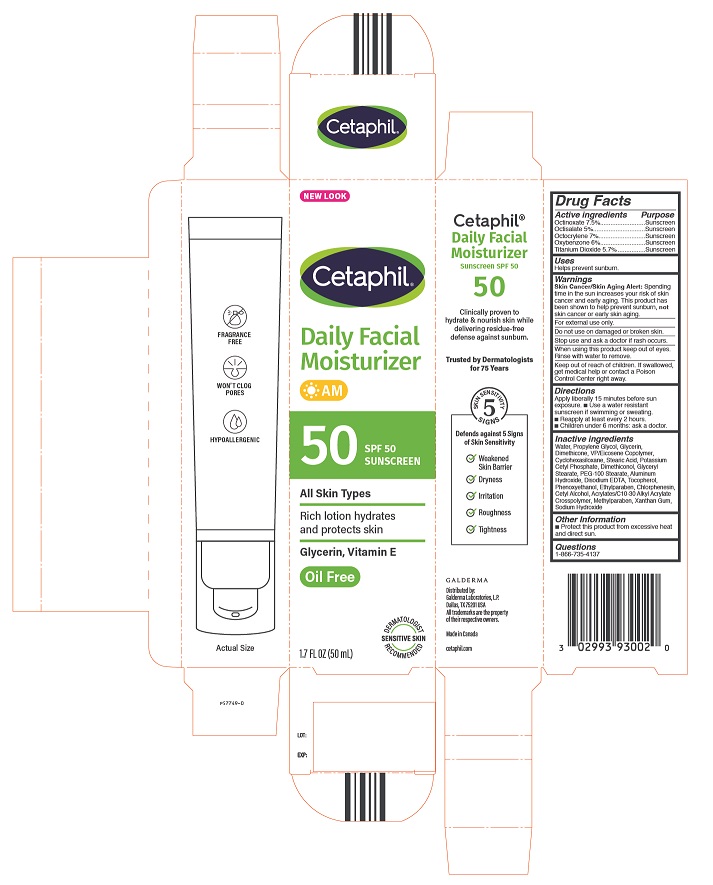
NEW LOOK
Cetaphil®
Daily Facial
Moisturizer
AM50 SPF 50
SunscreenAll Skin Types
Rich lotion hydrates
and protects skinGlycerin, Vitamin E
Oil Free
Dermatologist Recommended
Sensitive Skin
1.7 FL OZ (50 mL)Distributed by:
Galderma Laboratories, L.P.
Dallas, TX 75201 USA
All trademarks are the property of their respective owners.
Made in Canada
cetaphil.com
P57749-0 -
INGREDIENTS AND APPEARANCE
CETAPHIL DAILY FACIAL MOISTURIZER WITH SUNCREEN SPF 50
octinoxate, octisalate, octocrylene, oxybenzone, titanium dioxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0299-4930 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 70 mg in 1 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 60 mg in 1 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 57 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) GLYCERIN (UNII: PDC6A3C0OX) DIMETHICONE (UNII: 92RU3N3Y1O) VINYLPYRROLIDONE/EICOSENE COPOLYMER (UNII: 035MV9S1C3) Cyclomethicone 6 (UNII: XHK3U310BA) STEARIC ACID (UNII: 4ELV7Z65AP) Potassium Cetyl Phosphate (UNII: 03KCY6P7UT) Dimethiconol (40 Cst) (UNII: 343C7U75XW) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) EDETATE DISODIUM (UNII: 7FLD91C86K) TOCOPHEROL (UNII: R0ZB2556P8) Phenoxyethanol (UNII: HIE492ZZ3T) Ethylparaben (UNII: 14255EXE39) Chlorphenesin (UNII: I670DAL4SZ) Cetyl Alcohol (UNII: 936JST6JCN) Acrylates/C10-30 Alkyl Acrylate Crosspolymer (60000 Mpa.S) (UNII: 8Z5ZAL5H3V) Methylparaben (UNII: A2I8C7HI9T) Xanthan Gum (UNII: TTV12P4NEE) Sodium Hydroxide (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0299-4930-02 1 in 1 CARTON 01/01/2012 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/01/2012 Labeler - Galderma Laboratories, L.P. (047350186) Establishment Name Address ID/FEI Business Operations G Production Inc. 251676961 manufacture(0299-4930)