ANADA# 200-330. Approved by FDA.
For topical use on dogs and cats only.
Not for ophthalmic use.
CAUTION:
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
DESCRIPTION:
Animax Cream combines nystatin, neomycin sulfate, thiostrepton and triamcinolone acetonide.
Each gram contains:
nystatin................................................................................................................ 100,000 units
neomycin sulfate equivalent to neomycin base................................................................ 2.5 mg
thiostrepton ............................................................................................................. 2,500 units
triamcinolone acetonide ................................................................................................... 1 mg
in an aqueous non-irritating vanishing cream base of polyoxyethylene fatty alcohol ether, sorbitol solution, propylene glycol, white petrolatum, polyoxyl 40 stearate, glyceryl monostearate, titanium dioxide, citric acid, ethylenediamine, methylparaben, propylparaben.
ACTIONS:
-
- By virtue of its four active ingredients, Animax Cream (nystatin-neomycin sulfate-thiostrepton-triamcinolone acetonide cream USP) provides four basic therapeutic effects: anti-inflammatory, antipruritic, antifungal and antibacterial. Triamcinolone acetonide is a potent synthetic corticosteroid providing rapid and prolonged symptomatic relief on topical administration. Inflammation, edema, and pruritis promptly subside, and lesions are permitted to heal. Nystatin is the first well-tolerated antifungal antibiotic of dependable efficacy for the treatment of cutaneous infections caused by Candida albicans (Monilia). Nystatin is fungistatic in vitro against a variety of yeast and yeast-like fungi including many fungi pathogenic to animals. No appreciable activity is exhibited against bacteria.
Thiostrepton has a high order of activity against gram-positive organisms, including many which are resistant to other antibiotics; neomycin exerts antimicrobial action against a wide range of gram-positive and gram-negative bacteria. Together they provide comprehensive therapy against those organisms responsible for most superficial bacterial infections.
INDICATIONS:
-
- Animax Cream (nystatin-neomycin sulfate-thiostrepton-triamcinolone acetonide cream USP) is indicated in the management of dermatologic disorders in dogs and cats, characterized by inflammation and dry or exudative dermatitis, particularly those caused, complicated, or threatened by bacterial or candidal (Candida albicans) infections. It is also of value in eczematous dermatitis, contact dermatitis, and seborrheic dermatitis; and as an adjunct in the treatment of dermatitis due to parasitic infestation.
CONTRAINDICATIONS:
-
- Animax Cream should not be used ophthalmically.
WARNINGS:
-
- Animax Cream is indicated for use in dogs and cats only. Not for use in animals which are raised for food.
Absorption of triamcinolone acetonide through topical application and by licking may occur. Therefore animals should be observed closely for signs of polydipsia, polyuria and increased weight gain particularly when the preparation is used over large areas or for extended periods of time.
Clinical and experimental data have demonstrated that corticosteroids administered orally or by injection to animals may induce the first stage of parturition if used during the last trimester of pregnancy and may precipitate premature parturition followed by dystocia, fetal death, retained placenta and metritis.
Additionally, corticosteroids administered to dogs, rabbits and rodents during pregnancy have resulted in cleft palate in offspring. Corticosteroids administered to dogs during pregnancy have also resulted in other congenital anomalies including deformed forelegs, phocomelia and anasarca.
PRECAUTIONS:
-
- Animax Cream (Nystatin-Neomycin Sulfate- Thiostrepton-Triamcinolone Acetonide Cream USP) is not intended for the treatment of deep abscesses or deep-seated infections such as inflammation of the lymphatic vessels. Parenteral antibiotic therapy is indicated in these infections. Nystatin-Neomycin Sulfate-Thiostrepton-Triamcinolone Acetonide Cream USP has been extremely well tolerated. The occurrence of systemic reactions is rarely a problem with topical administration.
Sensitivity to neomycin may occur. If redness, irritation or swelling persists or increases, discontinue use. Do not use if pus is present since the drug may allow the infection to spread.
Avoid ingestion. Oral or parental use of corticosteroids, depending on dose, duration and specific steroid, may result in inhibition of endogenous steroid production following drug withdrawal.
SIDE EFFECTS:
-
- SAP and SGPT (ALT) enzyme elevations, polydipsia and polyuria have occurred following parenteral or systemic use of synthetic corticosteroids in dogs. Vomiting and diarrhea (occasionally bloody) have been observed in dogs. Cushing's syndrome in dogs has been reported in association with prolonged or repeated steroid therapy.
DOSAGE AND ADMINISTRATION:
-
- Frequency of administration is dependent on the severity of the condition. For mild inflammations, application may range from once daily to once a week; for severe conditions Animax Cream may be applied as often as 2 to 3 times daily, if necessary. Frequency of treatment may be decreased as improvement occurs.
Clean affected areas, removing any encrusted discharge or exudate. Apply Animax Cream (Nystatin-Neomycin Sulfate- Thiostrepton-Triamcinolone Acetonide Cream USP) sparingly in a thin film.
HOW SUPPLIED:
-
- Animax Cream (nystatin-neomycin sulfate-thiostrepton-triamcinolone acetonide cream USP) is supplied in tubes as follows:
7.5 gram tubes NDC 17033-123-75
15 gram tubes NDC 17033-123-15
STORAGE:
-
- Store at room temperature; avoid excessive heat (104°F).
TO OPEN:
-
- Use cap to puncture seal.
IMPORTANT:
-
- Do not use if seal has been punctured or is not visible.
MANUFACTURED FOR:
Dechra Veterinary Products
Overland Park, KS 66211
I123 DECHRA R07/08 #288
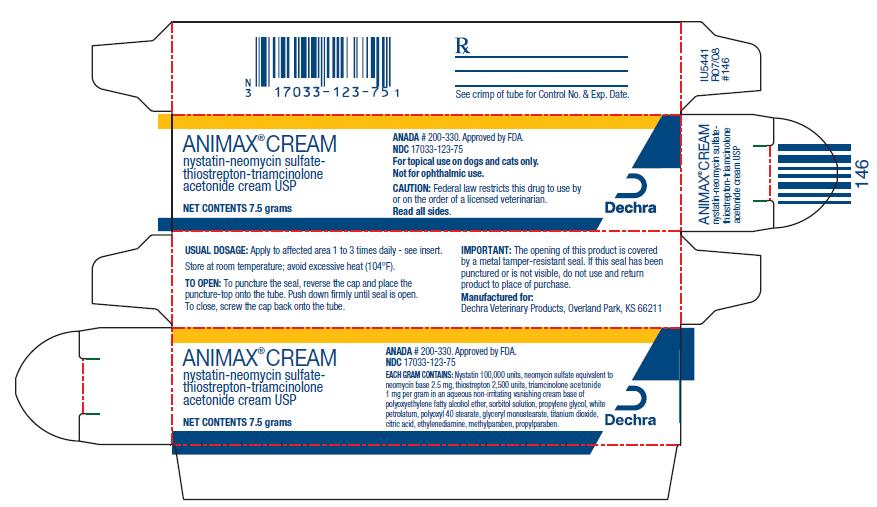
PRINCIPAL DISPLAY PANEL - 7.5 g Carton
ANIMAX® CREAM
nystatin-neomycin sulfate-thiostrepton-triamcinolone acetonide cream
NET CONTENTS 7.5 g
ANADA # 200-330. Approved by FDA
NDC 17033-123-75
PRINCIPAL DISPLAY PANEL - 7.5 g Container
-
- NET CONTENTS 7.5 g
NDC 17033-123-75
ANIMAX ® CREAM
nystatin-neomycin sulfate-thiostrepton-triamcinolone acetonide cream
ANADA # 200-330. Approved by FDA.