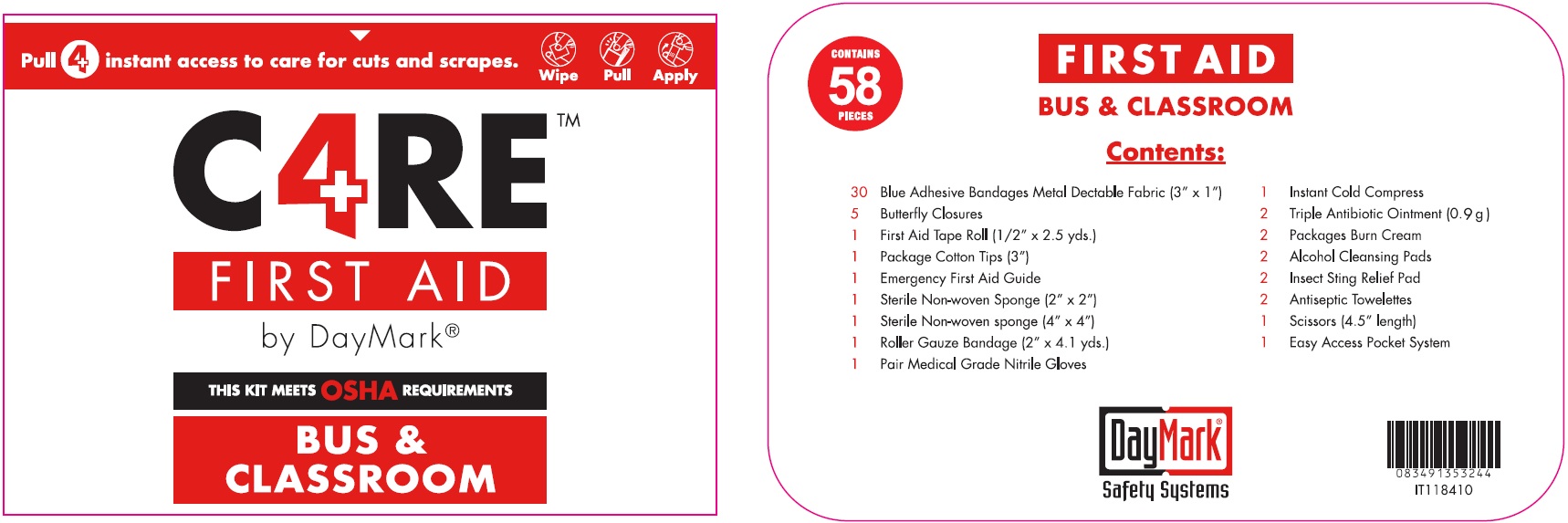
CARE BUS AND CLASSROOM YELLOW- bacitracin zinc, neomycin sulfate, polymyxin b sulfate, benzalkonium chloride, lidocaine hydrochloride, isopropyl alcohol, benzocaine, alcohol
CMC Group, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Kit Care4 Bus & Classroom Yellow
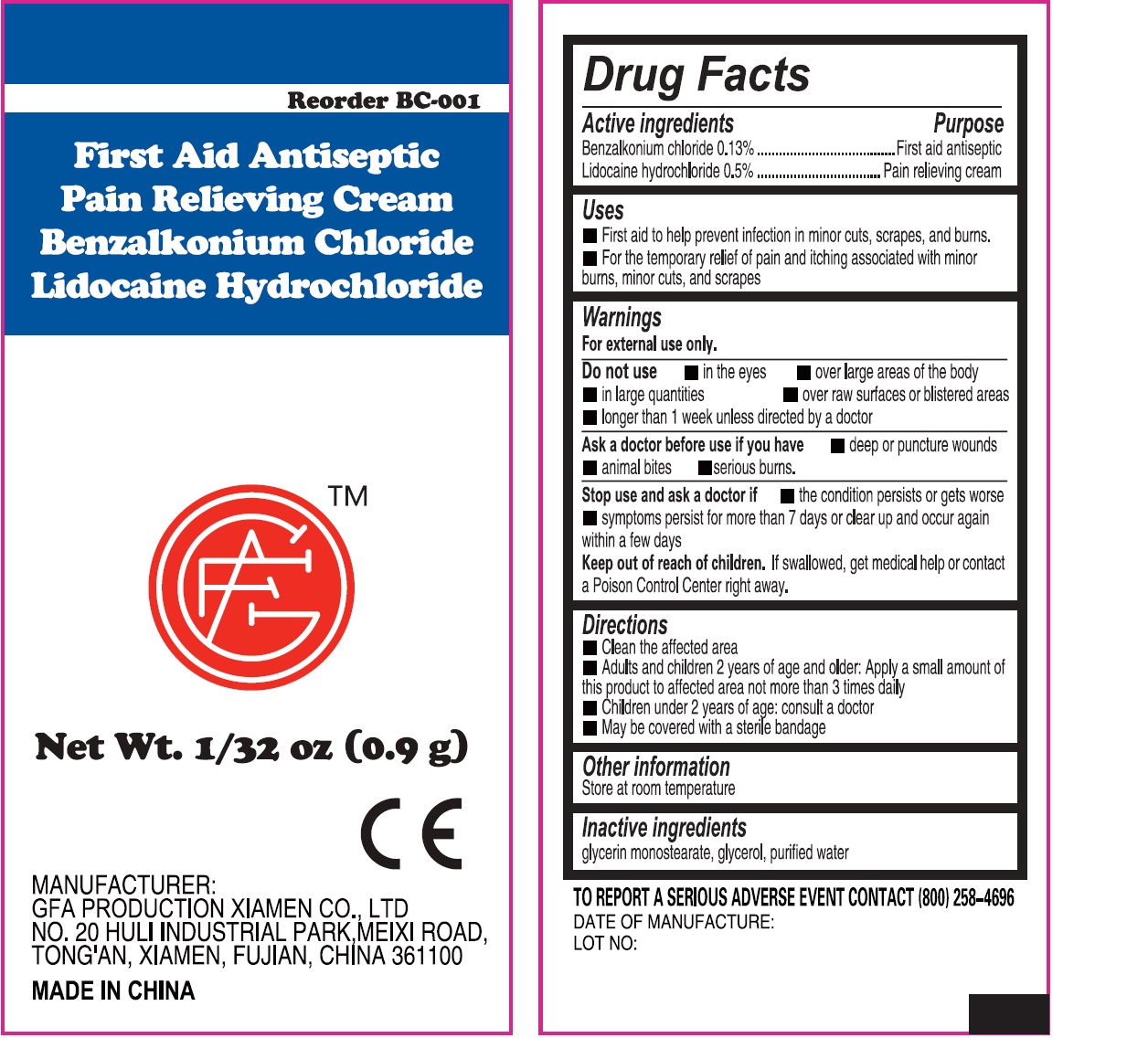
Active ingredients (in each gram)
Bacitracin zinc (bacitracin 400 units)
Neomycin sulfate (neomycin 3.5 mg)
Polymyxin B sulfate (polymyxin B 5,000 units)
Warnings
For external use only.
Do not use
• Do not use • in the eyes • over large areas of the body • if you are allergic to any of the ingredients
• longer than 1 week unless directed by a doctor.
Directions
• Clean the affected area. • Apply a small amount of this product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily. • May be covered with a sterile bandage.
Uses
- First aid to help prevent infection in minor cuts, scrapes, and burns.
- For the temporary relief of pain and itching associated with minor burns, minor cuts, and scrapes.
Warnings
For external use only.
Do not use
• in the eyes • over large areas of the body • in large quantities • over raw surfaces or blistered areas • longer than 1 week unless directed by a doctor
Directions
- Clean the affected area
- Adults and children 2 years of age and older: Apply a small amount of this product to affected area not more than 3 times daily
- Children under 2 years of age: consult a doctor
- May be covered with a sterile bandage
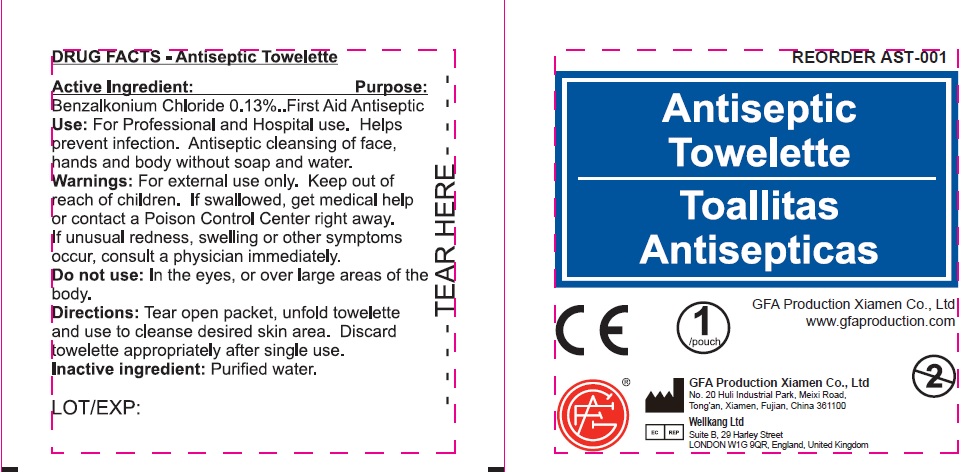
Warnings:
For external use only.
Flammable: keep away from fire or flame.
Use:
For Professional and Hospital use. Helps prevent infection. Antiseptic cleansing of face, hands and body without soap and water.
Warnings:
For external use only.
Directions:
Tear open packet, unfold towelette and use to cleanse desired skin area. Discard towelette appropriately after single use.
Use:
For the temporary relief of pain and itching associated with minor burns, scrapes and insect bites.
| CARE BUS AND CLASSROOM YELLOW
bacitracin zinc, neomycin sulfate, polymyxin b sulfate, benzalkonium chloride, lidocaine hydrochloride, isopropyl alcohol, benzocaine, alcohol kit |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - CMC Group, Inc. (005583328) |