Label: NEOMYCIN AND POLYMYXIN B SULFATES AND GRAMICIDIN- neomycin sulfate, polymyxin b sulfate and gramicidin solution/ drops
-
Contains inactivated NDC Code(s)
NDC Code(s): 21695-528-10 - Packager: Rebel Distributors Corp
- This is a repackaged label.
- Source NDC Code(s): 24208-790
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 3, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Neomycin and Polymyxin B Sulfates and Gramicidin Ophthalmic Solution USP is a sterile antimicrobial solution for ophthalmic use.
EACH mL CONTAINS: ACTIVES: Neomycin Sulfate, (equivalent to 1.75 mg neomycin base), Polymyxin B Sulfate equal to 10,000 Polymyxin B units, Gramicidin, 0.025 mg; INACTIVES: Sodium Chloride, Alcohol (0.5%), Poloxamer 188, Propylene Glycol, Purified Water. Hydrochloric Acid and/ or Ammonium Hydroxide may be added to adjust pH (4.7- 6.0).
PRESERVATIVE ADDED: Thimerosal 0.001%.
Neomycin Sulfate is the sulfate salt of neomycin B and C, which are produced by the growth of Streptomyces fradiae Waksman (Fam. Streptomycetaceae). It has a potency equivalent of not less than 600 micrograms of neomycin base per milligram, calculated on an anhydrous basis.
The structural formulae are:
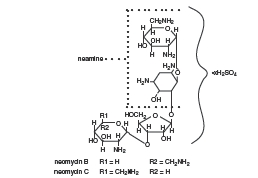
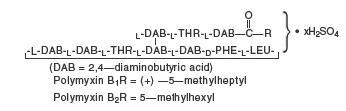
Polymyxin B Sulfate is the sulfate salt of polymyxin Bl and B2 which are produced by the growth of Bacillus polymyxa (Prazmowski) Migula (Fam. Bacillaceae). It has a potency of not less than 6,000 polymyxin B units per milligram, calculated on an anhydrous basis. The structural formulae are:
Gramicidin (also called gramicidin D) is a mixture of three pairs of antibacterial substances (Gramicidin A, B and C) produced by the growth of Bacillusbrevis Dubos (Fam. Bacillaceae). It has a potency of not less than 900 mcg of standard gramicidin per mg. The structural formulae are:
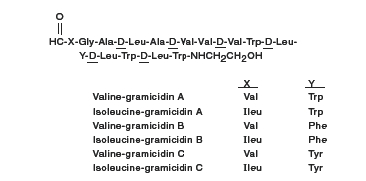
-
CLINICAL PHARMACOLOGY
A wide range of antibacterial action is provided by the overlapping spectra of neomycin, polymyxin B sulfate, and gramicidin.
Neomycin is bactericidal for many gram-positive and gram-negative organisms. It is an aminoglycoside antibiotic which inhibits protein synthesis by binding with ribosomal RNA and causing misreading of the bacterial genetic code.
Polymyxin B is bactericidal for a variety of gram-negative organisms. It increases the permeability of the bacterial cell membrane by interacting with the phospholipid components of the membrane.
Gramicidin is bactericidal for a variety of gram-positive organisms. It increases the permeability of the bacterial cell membrane to inorganic cations by forming a network of channels through the normal lipid bilayer of the membrane.
Microbiology: Neomycin sulfate, polymyxin B sulfate and gramicidin together are considered active against the following microorganisms:
Staphylococcus aureus, streptococci, including Streptococcus pneumoniae, Escherichia coli, Haemophilus influenzae, Klebsiella-Enterobacter species, Neisseria species and Pseudomonas aeruginosa. The product does not provide adequate coverage against Serratia marcescens.
-
INDICATIONS AND USAGE
Neomycin and Polymyxin B Sulfates and Gramicidin Ophthalmic Solution is indicated for the topical treatment of superficial infections of the external eye and its adnexa caused by susceptible bacteria. Such infections encompass conjunctivitis, keratitis and keratoconjunctivitis, blepharitis and blepharoconjunctivitis.
- CONTRAINDICATIONS
-
WARNINGS
NOT FOR INJECTION INTO THE EYE. This product should never be directly introduced into the anterior chamber of the eye or injected subconjunctivally.
Topical antibiotics, particularly neomycin sulfate, may cause cutaneous sensitization. A precise incidence of hypersensitivity reactions (primarily skin rash) due to topical antibiotics is not known.
The manifestations of sensitization to topical antibiotics are usually itching, reddening and edema of the conjunctiva and eyelid. A sensitization reaction may manifest simply as a failure to heal. During long-term use of topical antibiotic products, periodic examination for such signs is advisable, and the patient should be told to discontinue the product if they are observed. Symptoms usually subside quickly on withdrawing the medication.
Applications of products containing these ingredients should be avoided for the patient thereafter. (See PRECAUTIONS, General)
-
PRECAUTIONS
General
As with other antibiotic preparations, prolonged use of this product may result in overgrowth of nonsusceptible organisms including fungi. If superinfection occurs, appropriate measures should be initiated.
Bacterial resistance to this product may also develop. If purulent discharge, inflammation or pain becomes aggravated, the patient should discontinue use of the medication and consult a physician.
There have been reports of bacterial keratitis associated with the use of topical ophthalmic products in multiple-dose containers which have been inadvertently contaminated by patients, most of whom had a concurrent corneal disease or a disruption of the ocular epithelial surface (see PRECAUTIONS: Information for Patients).
Allergic cross-reactions may occur which could prevent the use of any or all of the following antibiotics for the treatment of future infections: kanamycin, paromomycin, streptomycin, and possibly gentamicin.
Information for patients
Patients should be instructed to avoid allowing the tip of the dispensing container to contact the eye, eyelid, fingers, or any other surface. The use of this product by more than one person may spread infection.
Patients should also be instructed that ocular products, if handled improperly, can become contaminated by common bacteria known to cause ocular infections. Serious damage to the eye and subsequent loss of vision may result from using contaminated products (see PRECAUTIONS: General).
If the condition persists or gets worse, or if a rash or other allergic reaction develops, the patient should be advised to stop use and consult a physician. Do not use this product if you are allergic to any of the listed ingredients.
Keep tightly closed when not in use. Keep out of reach of children.
Carcinogenesis, mutagenesis, impairment of fertility
Long-term studies in animals to evaluate carcinogenic or mutagenic potential have not been conducted with polymyxin B sulfate or gramicidin. Treatment of cultured lymphocytes in vitro with neomycin increased the frequency of chromosome aberrations at the highest concentration (80 mcg/mL) tested. However, the effects of neomycin on carcinogenesis and mutagenesis in humans are unknown.
Polymyxin B has been reported to impair the motility of equine sperm, but its effects on male or female fertility are unknown.
Pregnancy
Teratogenic effects
Pregnancy Category C. Adequate animal reproductive studies have not been conducted with neomycin, polymyxin B or gramicidin. It is also not known whether this product can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. This product should be given to a pregnant woman only if clearly needed.
-
ADVERSE REACTIONS
Adverse reactions have occurred with the anti-infective components of this product. The exact incidence is not known. Reactions occurring most often are allergic reactions including itching, swelling, and conjunctival erythema (see WARNINGS). More serious hypersensitivity reactions, including anaphylaxis, have been reported rarely.
Local irritation on instillation has also been reported.
- DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- STORAGE
- MANUFACTURER INFORMATION
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
NEOMYCIN AND POLYMYXIN B SULFATES AND GRAMICIDIN
neomycin sulfate, polymyxin b sulfate and gramicidin solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:21695-528(NDC:24208-790) Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Neomycin Sulfate (UNII: 057Y626693) (Neomycin - UNII:I16QD7X297) Neomycin Sulfate 1.75 mg in 1 mL Polymyxin B Sulfate (UNII: 19371312D4) (Polymyxin B - UNII:J2VZ07J96K) Polymyxin B Sulfate 10000 mg in 1 mL Gramicidin (UNII: 5IE62321P4) (Gramicidin - UNII:5IE62321P4) Gramicidin 0.025 mg in 1 mL Inactive Ingredients Ingredient Name Strength Alcohol (UNII: 3K9958V90M) AMMONIA (UNII: 5138Q19F1X) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) Hydrochloric Acid (UNII: QTT17582CB) POLOXAMER 188 (UNII: LQA7B6G8JG) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Water (UNII: 059QF0KO0R) Sodium Chloride (UNII: 451W47IQ8X) Thimerosal (UNII: 2225PI3MOV) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21695-528-10 1 in 1 CARTON 1 10 mL in 1 BOTTLE, DROPPER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA064047 01/31/1996 Labeler - Rebel Distributors Corp (118802834) Establishment Name Address ID/FEI Business Operations Rebel Distributors Corp 118802834 RELABEL, REPACK


