Label: AMPROMED FOR CALVES- amprolium solution
- NDC Code(s): 61133-8251-3, 61133-8251-4
- Packager: Bimeda Inc.
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Animal Drug Application
Drug Label Information
Updated July 5, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
INDICATIONS & USAGE
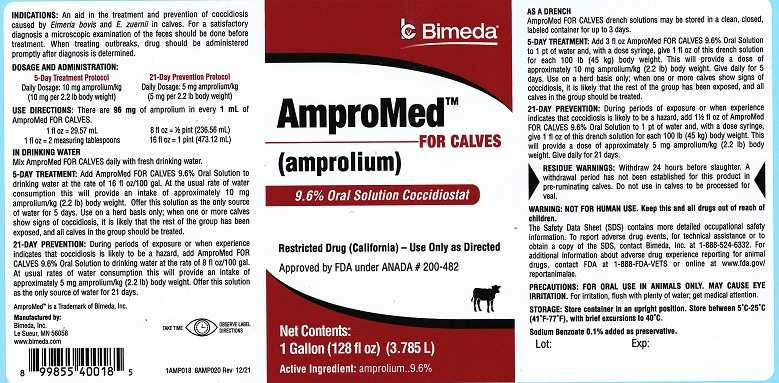
AmproMed™ FOR CALVES
(amprolium)
9.6% Oral Solution Coccidiostat
Restricted Drug (California) – Use Only as Directed
Approved by FDA under ANADA # 200-482
Net Contents:
1 Gallon (128 fl oz) (3.785 L)
Active Ingredient: amprolium..............9.6%
INDICATIONS: An aid in the treatment and prevention of coccidiosis caused by Eimeria bovis and E. zuernii in calves. For a satisfactory diagnosis a microscopic examination of the feces should be done before treatment. When treating outbreaks, drug should be administered promptly after diagnosis is determined.
-
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION:
5-Day Treatment Protocol
21-Day Prevention Protocol
Daily Dosage: 10 mg amprolium/kg
(10 mg per 2.2 lb body weight)
Daily Dosage: 5 mg amprolium/kg
(5 mg per 2.2 lb body weight)
USE DIRECTIONS: There are 96 mg of Amprolium in every 1 mL of AmproMed FOR CALVES
1 fl oz = 29.57 mL
1 fl oz = 2 measuring tablespoons
8 fl oz = ½ pint (236.56 mL)
16 fl oz = 1 pint (473.12 mL)
IN DRINKING WATER
Mix AmproMed FOR CALVES daily with fresh drinking water.
5-DAY TREATMENT: Add AMPROMED FOR CALVES 9.6% Oral Solution to drinking water at the rate of 16 fl oz/100 gallon. At the usual rate of water consumption this will provide an intake of approximately 10 mg amprolium/kg (2.2 lb) body weight. Offer this solution as the only source of water for 5 days. Use on a herd basis only; when one or more calves show signs of coccidiosis, it is likely that the rest of the group has been exposed, and all calves in the group should be treated.
21-DAY PREVENTION: During periods of exposure or when experience indicates that coccidiosis is likely to be a hazard, add AMPROMED FOR CALVES 9.6% Oral Solution to drinking water at the rate of 8 fl oz/100 gal. At usual rates of water consumption, this will provide an intake of approximately 5 mg amprolium/kg (2.2 lb) body weight. Offer this solution as the only source of water for 21 days.
AS A DRENCH: AMPROMED FOR CALVES drench solutions may be stored in a clean, closed labeled container for up to 3 days.5-DAY TREATMENT: Add 3 fl oz AMPROMED FOR CALVES 9.6% Oral Solution to 1 pt of water and, with a dose syringe, give 1 fl oz of this drench solution for each 100 lb (45 kg) body weight. This will provide a dose of approximately 10 mg amprolium/kg (2.2 lb) body weight. Give daily for 5 days. Use on a herd basis only; when one or more calves show signs of coccidiosis, it is likely that the rest of the group has been exposed, and all calves should be treated.
21-DAY PREVENTION: During periods of exposure or when experience indicates that coccidiosis is likely to be a hazard, add 1 1/2 fl oz of AMPROMED FOR CALVES 9.6% Oral Solution to 1 pt of water and, with a dose syringe, give 1 fl oz of this solution for each 100 lb (45 kg) body weight. This will provide a dose of approximately 5 mg amprolium/kg (2.2 lb) body weight. Give daily for 21 days.
- RESIDUE WARNING
- WARNINGS
- PRECAUTIONS
-
ADVERSE REACTIONS
The Safety Data Sheet (SDS) contains more detailed occupational safety information. To report adverse drug events, for technical assistance or to obtain a copy of the SDS contact Bimeda, Inc. at 1-888-524-6332. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at www.fda.gov/reportanimalae.
- STORAGE AND HANDLING
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
AMPROMED FOR CALVES
amprolium solutionProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:61133-8251 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMPROLIUM (UNII: 95CO6N199Q) (AMPROLIUM ION - UNII:H2T307KMZR) AMPROLIUM ION 96 mg in 1 mL Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61133-8251-3 3785 mL in 1 JUG 2 NDC:61133-8251-4 473 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200482 07/30/2012 Labeler - Bimeda Inc. (060492923) Registrant - Bimeda, Inc. (060492923) Establishment Name Address ID/FEI Business Operations Bimeda, Inc. 060492923 manufacture