Label: PANCREVED- pancreatic enzyme tablet
- NDC Code(s): 50989-312-51, 50989-312-52
- Packager: Vedco, INC
- Category: PRESCRIPTION ANIMAL DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 23, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
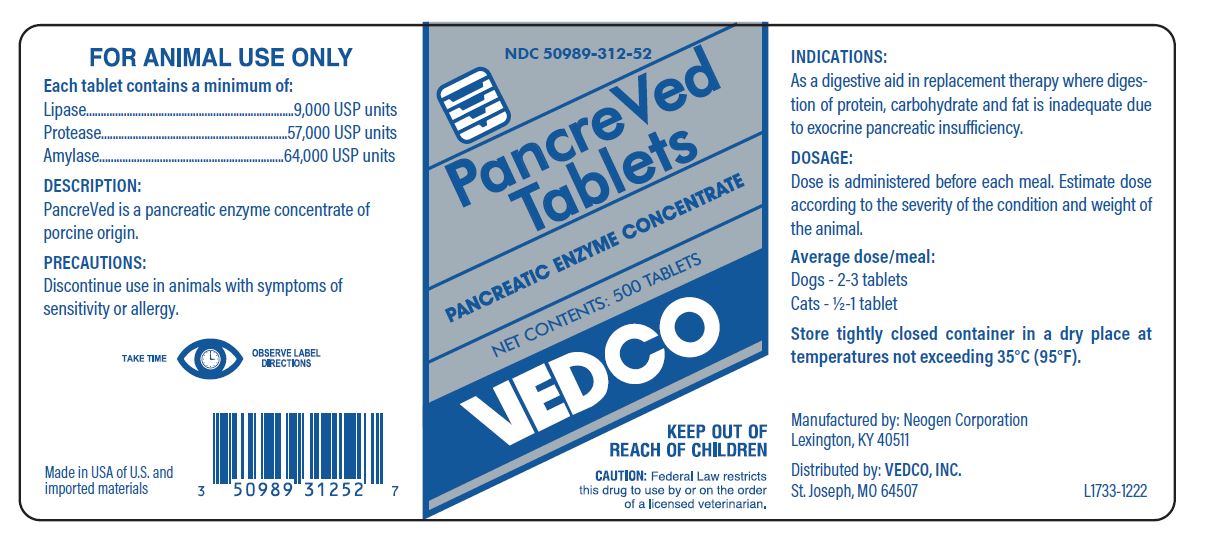
- FOR ANIMAL USE ONLY
- Each tablet contains a minimum of:
- TAKE TIME OBSERVE LABEL DIRECTIONS
- DOSAGE:
- INDICATIONS:
- PRECAUTIONS:
- Store tightly closed container in a dry place at temperature not exceeding 35°C (95°F).
- PRINCIPAL DISPLAY PANEL - VEDCO PancreVed Tablets 100 Tablet Bottle
- PRINCIPAL DISPLAY PANEL - VEDCO PancreVed Tablets 500 Tablet Bottle
-
INGREDIENTS AND APPEARANCE
PANCREVED
pancreatic enzyme tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:50989-312 Route of Administration oral Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PANCRELIPASE LIPASE (UNII: 8MYC33932O) (PANCRELIPASE LIPASE - UNII:8MYC33932O) PANCRELIPASE LIPASE 9000 [iU] PANCRELIPASE AMYLASE (UNII: YOJ58O116E) (PANCRELIPASE AMYLASE - UNII:YOJ58O116E) PANCRELIPASE AMYLASE 64000 [iU] PANCRELIPASE PROTEASE (UNII: 3560D81V50) (PANCRELIPASE PROTEASE - UNII:3560D81V50) PANCRELIPASE PROTEASE 57000 [iU] Product Characteristics Color brown Score no score Shape ROUND Size 11mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50989-312-51 100 in 1 BOTTLE, PLASTIC 2 NDC:50989-312-52 500 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 11/08/2012 Labeler - Vedco, INC (021634266) Establishment Name Address ID/FEI Business Operations Scientific Protein Laboratories LLC 065240319 analysis, api manufacture Establishment Name Address ID/FEI Business Operations ALI Pharmaceutical Manufacturing, LLC 080861914 api manufacture Establishment Name Address ID/FEI Business Operations Neogen Corporation 042125879 label, analysis, manufacture