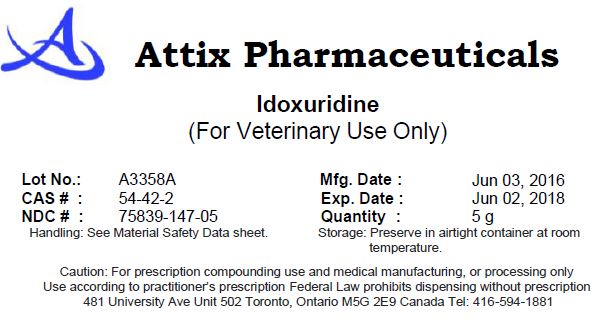
IDOXURIDINE- idoxuridine powder
Attix Pharmaceuticals
----------
Idoxuridine
| IDOXURIDINE
idoxuridine powder |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Attix Pharmaceuticals (248276599) |
Revised: 12/2021
Document Id: d291f70b-0ef6-6109-e053-2a95a90ab879
Set id: 64f1cc84-b369-4463-8362-da1f849d4438
Version: 7
Effective Time: 20211207
Attix Pharmaceuticals