Label: NOREPINEPHRINE BITARTRATE injection, solution
- NDC Code(s): 0338-0108-20, 0338-0112-20, 0338-0116-20
- Packager: Baxter Healthcare Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use NOREPINEPHRINE BITARTRATE IN DEXTROSE injection safely and effectively. See full prescribing information for NOREPINEPHRINE BITARTRATE IN DEXTROSE injection.
NOREPINEPHRINE BITARTRATE IN DEXTROSE injection, for intravenous use
Initial U.S. Approval: 1950INDICATIONS AND USAGE
Norepinephrine Bitartrate in Dextrose Injection is a catecholamine indicated for restoration of blood pressure in adult patients with acute hypotensive states. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Injection: 250-mL single dose-containers with (3)
- •
- 4 mg equivalent of norepinephrine (16 mcg/mL) in 5% dextrose.
- •
- 8 mg equivalent of norepinephrine (32 mcg/mL) in 5% dextrose.
- •
- 16 mg equivalent of norepinephrine (64 mcg/mL) in 5% dextrose.
CONTRAINDICATIONS
- •
- None. (4)
WARNINGS AND PRECAUTIONS
- •
- Tissue Ischemia: Infuse Norepinephrine Bitartrate in Dextrose Injection into a large vein. To prevent sloughing and necrosis in areas in with extravasation, infiltrate the area with an adrenergic blocking agent in saline. (5.1)
- •
- Hypotension After Abrupt Discontinuation: Gradually taper a norepinephrine infusion to prevent hypotension. (5.2)
- •
- Cardiac Arrhythmias: Norepinephrine Bitartrate in Dextrose Injection may cause arrhythmias. Monitor cardiac function in patients with underlying heart disease. (5.3)
ADVERSE REACTIONS
Serious adverse reactions are described in greater detail in other sections [see Warnings and Precautions (5.1, 5.2, & 5.3)].
Most common adverse reactions are hypertension, bradycardia, ischemic injury, anxiety, headache, respiratory difficulty, pulmonary edema, and extravasation necrosis at injection site. (6)To report SUSPECTED ADVERSE REACTIONS, contact Baxter Healthcare at 1-866-888-2472 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- •
- Monoamine oxidase inhibitors (MAOI) or tricyclic antidepressants of the triptyline or imipramine types may result in hypertension. (7.1, 7.2)
- •
- Antidiabetics: Norepinephrine can decrease insulin sensitivity and raise blood glucose (7.3)
- •
- Cyclopropane and halothane anesthetics increase cardiac autonomic irritability. (7.4)
USE IN SPECIFIC POPULATIONS
- •
- Elderly patients may be at greater risk of developing adverse reactions (8.5)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions
2.2 Dosage
2.3 Drug Incompatibilities
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Tissue Ischemia
5.2 Hypotension after Abrupt Discontinuation
5.3 Cardiac Arrhythmias
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
7.1 MAO-Inhibiting Drugs
7.2 Tricyclic Antidepressants
7.3 Antidiabetics
7.4 Halogenated Anesthetics
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions
Correct Hypovolemia
Address hypovolemia before initiation of Norepinephrine Bitartrate in Dextrose Injection therapy. If the patient does not respond to therapy, suspect occult hypovolemia [see Warnings and Precautions (5.1)].
Administration
Norepinephrine Bitartrate in Dextrose Injection is a ready to administer product that requires no further dilution prior to infusion. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use the solution if its color is pinkish or darker than slightly yellow or if it contains a precipitate.
Infuse Norepinephrine Bitartrate in Dextrose Injection into a large vein. Avoid infusions into the veins of the leg in the elderly or in patients with occlusive vascular disease of the legs [see Warnings and Precautions (5.1)]. Avoid using a catheter-tie-in technique.
The choice of appropriate concentration of Norepinephrine Bitartrate in Dextrose Injection depends on clinical fluid volume requirements. Use higher concentration solutions in patients requiring fluid restriction.
Discontinuation
When discontinuing the infusion, reduce the flow rate gradually. Avoid abrupt withdrawal.
2.2 Dosage
After an initial dosage of 8 to 12 mcg per minute via intravenous infusion, assess patient response and adjust dosage to maintain desired hemodynamic effect. Monitor blood pressure every two minutes until the desired hemodynamic effect is achieved, and then monitor blood pressure every five minutes for the duration of the infusion.
Typical maintenance intravenous dosage is 2 to 4 mcg per minute.
-
3 DOSAGE FORMS AND STRENGTHS
Injection: Norepinephrine bitartrate in 5% dextrose is a colorless to slightly yellow solution for intravenous infusion, supplied in 250-mL single dose containers as:
- •
- 4 mg equivalent of norepinephrine (16 mcg/mL).
- •
- 8 mg equivalent of norepinephrine (32 mcg/mL).
- •
- 16 mg equivalent of norepinephrine (64 mcg/mL).
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Tissue Ischemia
Administration of Norepinephrine Bitartrate in Dextrose Injection to patients who are hypotensive from hypovolemia can result in severe peripheral and visceral vasoconstriction, decreased renal perfusion and reduced urine output, tissue hypoxia, lactic acidosis, and reduced systemic blood flow despite “normal” blood pressure. Address hypovolemia prior to initiating Norepinephrine Bitartrate in Dextrose Injection [see Dosage and Administration (2.1)]. Avoid Norepinephrine Bitartrate in Dextrose Injection in patients with mesenteric or peripheral vascular thrombosis, as this may increase ischemia and extend the area of infarction.
Gangrene of the extremities has occurred in patients with occlusive or thrombotic vascular disease or who received prolonged or high dose infusions. Monitor for changes to the skin of the extremities in susceptible patients.
Extravasation of Norepinephrine Bitartrate in Dextrose Injection may cause necrosis and sloughing of surrounding tissue. To reduce the risk of extravasation, infuse into a large vein, check the infusion site frequently for free flow, and monitor for signs of extravasation [see Dosage and Administration (2.1)].
Emergency Treatment of Extravasation
To prevent sloughing and necrosis in areas in which extravasation has occurred, infiltrate the ischemic area as soon as possible, using a syringe with a fine hypodermic needle with 5 to 10 mg of phentolamine mesylate in 10 to 15 mL of 0.9% Sodium Chloride Injection in adults.
Sympathetic blockade with phentolamine causes immediate and conspicuous local hyperemic changes if the area is infiltrated within 12 hours.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described in greater detail in other sections:
- •
- Tissue Ischemia [see Warnings and Precautions (5.1)]
- •
- Hypotension [see Warnings and Precautions (5.2)]
- •
- Cardiac Arrhythmias [see Warnings and Precautions (5.3)]
The most common adverse reactions are hypertension and bradycardia.
The following adverse reactions can occur:
Nervous system disorders: Anxiety, headache
Respiratory disorders: Respiratory difficulty, pulmonary edema
General disorders and administration site conditions: Extravasation, injection site necrosis [see Warnings and Precautions (5.1)].
-
7 DRUG INTERACTIONS
7.1 MAO-Inhibiting Drugs
Co-administration of Norepinephrine Bitartrate in Dextrose Injection with monoamine oxidase (MAO) inhibitors or other drugs with MAO-inhibiting properties (e.g., linezolid) can cause severe, prolonged hypertension.
If administration of Norepinephrine Bitartrate in Dextrose Injection cannot be avoided in patients who recently have received any of these drugs and in whom, after discontinuation, MAO activity has not yet sufficiently recovered, monitor for hypertension.
7.2 Tricyclic Antidepressants
Co-administration of Norepinephrine Bitartrate in Dextrose Injection with tricyclic antidepressants (including amitriptyline, nortriptyline, protriptyline, clomipramine, desipramine, imipramine) can cause severe, prolonged hypertension. If administration of Norepinephrine Bitartrate in Dextrose Injection cannot be avoided in these patients, monitor for hypertension.
7.3 Antidiabetics
Norepinephrine Bitartrate in Dextrose Injection can decrease insulin sensitivity and raise blood glucose. Monitor glucose and consider dosage adjustment of antidiabetic drugs.
7.4 Halogenated Anesthetics
Concomitant use of Norepinephrine Bitartrate in Dextrose Injection with halogenated anesthetics (e.g., cyclopropane, desflurane, enflurane, isoflurane, and sevoflurane) may lead to ventricular tachycardia or ventricular fibrillation. Monitor cardiac rhythm in patients receiving concomitant halogenated anesthetics.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Limited published data consisting of a small number of case reports and multiple small trials involving the use of norepinephrine in pregnant women at the time of delivery have not identified an increased risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. There are risks to the mother and fetus from hypotension associated with septic shock, myocardial infarction and stroke which are medical emergencies in pregnancy and can be fatal if left untreated. (see Clinical Considerations). In animal reproduction studies, using high doses of intravenous norepinephrine resulted in lowered maternal placental blood flow. Clinical relevance to changes in the human fetus is unknown since the average maintenance dose is ten times lower (see Data). Increased fetal reabsorptions were observed in pregnant hamsters after receiving daily injections at approximately 2 times the maximum recommended dose on a mg/m3 basis for four days during organogenesis (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Hypotension associated with septic shock, myocardial infarction, and stroke are medical emergencies in pregnancy which can be fatal if left untreated. Delaying treatment in pregnant women with hypotension associated with septic shock, myocardial infarction and stroke may increase the risk of maternal and fetal morbidity and mortality. Life-sustaining therapy for the pregnant woman should not be withheld due to potential concerns regarding the effects of norepinephrine on the fetus.
Data
Animal Data
A study in pregnant sheep receiving high doses of intravenous norepinephrine (40 mcg/min, at approximately 10 times the average maintenance dose of 2-4 mcg/min in human, on a mg/kg basis) exhibited a significant decrease in maternal placental blood flow. Decreases in fetal oxygenation, urine and lung liquid flow were also observed.
Norepinephrine administration to pregnant rats on Gestation Day 16 or 17 resulted in cataract production in rat fetuses.
In hamsters, an increased number of resorptions (29.1% in study group vs. 3.4% in control group), fetal microscopic liver abnormalities and delayed skeletal ossification were observed at approximately 2 times the maximum recommended intramuscular or subcutaneous dose (on a mg/m2 basis at a maternal subcutaneous dose of 0.5 mg/kg/day from Gestation Day 7-10).
8.2 Lactation
Risk Summary
There are no data on the presence of norepinephrine in either human or animal milk, the effects on the breastfed infant, or the effects on milk production. Clinically relevant exposure to the infant is not expected based on the short half-life and poor oral bioavailability of norepinephrine.
8.5 Geriatric Use
Clinical studies of norepinephrine did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Avoid administration of Norepinephrine Bitartrate in Dextrose Injection into the veins in the leg in elderly patients [see Warnings and Precautions (5.1)].
-
10 OVERDOSAGE
Overdosage with Norepinephrine Bitartrate in Dextrose Injection may result in headache, severe hypertension, reflex bradycardia, marked increase in peripheral resistance, and decreased cardiac output.
In case of overdosage, discontinue Norepinephrine Bitartrate in Dextrose Injection until the condition of the patient stabilizes.
-
11 DESCRIPTION
Norepinephrine Bitartrate in Dextrose Injection contains norepinephrine, a sympathomimetic amine. Norepinephrine is sometimes referred to as l-arterenol/Levarterenol or l-norepinephrine which differs from epinephrine by the absence of a methyl group on the nitrogen atom.
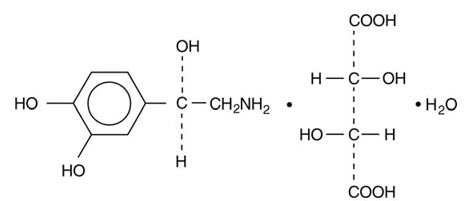
Chemically, norepinephrine bitartrate monohydrate is (-)-α-(aminomethyl)-3,4-dihydroxybenzyl alcohol tartrate (1:1) (salt) monohydrate and has the following structural formula:
Norepinephrine is sparingly soluble in water, very slightly soluble in alcohol and ether, and readily soluble in acids.
Norepinephrine Bitartrate in Dextrose Injection is supplied as a sterile aqueous solution administered by intravenous infusion. Each mL contains the equivalent of 16, 32, or 64 micrograms of norepinephrine base supplied as 31.90, 63.80, and 127.60 micrograms per mL of norepinephrine bitartrate monohydrate. It contains dextrose monohydrate (50 mg/mL) and may contain hydrochloric acid and/or sodium hydroxide for pH adjustment. It has a target pH of 3.7. The air in the containers has been displaced by nitrogen gas.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Norepinephrine is a peripheral vasoconstrictor (alpha-adrenergic action) and an inotropic stimulator of the heart and dilator of coronary arteries (beta-adrenergic action).
12.2 Pharmacodynamics
The primary pharmacodynamic effects of norepinephrine are cardiac stimulation and vasoconstriction. Cardiac output is generally unaffected, although it can be decreased, and total peripheral resistance is also elevated. The elevation in resistance and pressure result in reflex vagal activity, which slows the heart rate and increases stroke volume. The elevation in vascular tone or resistance reduces blood flow to the major abdominal organs as well as to skeletal muscle. Coronary blood flow is substantially increased secondary to the indirect effects of alpha stimulation. After intravenous administration, a pressor response occurs rapidly and reaches steady state within 5 minutes. The pharmacologic actions of norepinephrine are terminated primarily by uptake and metabolism in sympathetic nerve endings. The pressor action stops within 1-2 minutes after the infusion is discontinued.
12.3 Pharmacokinetics
Absorption
Following initiation of intravenous infusion, the steady state plasma concentration is achieved in 5 min.
Distribution
Plasma protein binding of norepinephrine is approximately 25%. It is mainly bound to plasma albumin and to a smaller extent to prealbumin and alpha 1-acid glycoprotein. The volume of distribution is 8.8 L. Norepinephrine localizes mainly in sympathetic nervous tissue. It crosses the placenta but not the blood-brain barrier.
Elimination
The mean half-life of norepinephrine is approximately 2.4 min. The average metabolic clearance is 3.1 L/min.
Metabolism
Norepinephrine is metabolized in the liver and other tissues by a combination of reactions involving the enzymes catechol-O-methyltransferase (COMT) and monoamine oxidase (MAO). The major metabolites are normetanephrine and 3-methoxy-4-hydroxy mandelic acid (vanillylmandelic acid, VMA), both of which are inactive. Other inactive metabolites include 3-methoxy-4-hydroxyphenylglycol, 3,4-dihydroxymandelic acid, and 3,4-dihydroxyphenylglycol.
Excretion
Norepinephrine metabolites are excreted in urine primarily as the sulfate conjugates and, to a lesser extent, as the glucuronide conjugates. Only small quantities of norepinephrine are excreted unchanged.
- 13 NONCLINICAL TOXICOLOGY
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Norepinephrine Bitartrate in Dextrose Injection for intravenous infusion is a colorless to slightly yellow solution available in single-dose, ready-to-use containers in an amber/foil overwrap. Each 250 mL of Norepinephrine Bitartrate in Dextrose Injection , 4 mg/250 mL, 8 mg/250 mL, and 16 mg/250 mL contains the equivalent of 4 mg, 8 mg, and 16 mg of norepinephrine, respectively (provided as norepinephrine bitartrate monohydrate). Norepinephrine Bitartrate in Dextrose Injection is available in the following:
Product Description
NDC Number
Twenty containers of 4 mg/250 mL
NDC 0338-0112-20
Twenty containers of 8 mg/250 mL
NDC 0338-0108-20
Twenty containers of 16 mg/250 mL
NDC 0338-0116-20
Store at room temperature [20°C to 25°C (68°F to 77°F)], excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Protect from light. Once the overwrap is removed, the bag can be stored at room temperature for up to 30 days.
-
17 PATIENT COUNSELING INFORMATION
Risk of Tissue Damage
Advise the patient, family, or caregiver to report signs of extravasation urgently [see Warnings and Precautions (5.1)].
- SPL UNCLASSIFIED SECTION
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
EZPE7748
NDC 0338-0112-20250 mL
Norepinephrine
Bitartrate in 5% Dextrose Injection4 mg / 250 mL
(16 mg / mL)
For Intravenous Infusion OnlyEach 100mL of sterile, nonpyrogenic solution contains: Norepinephrine
Bitartrate Monohydrate USP equivalent to 1.6 mg norepinephrine and
5 g Dextrose Monohydrate in Water for Injection, USP. May contain
hydrochloric acid and/or sodium hydroxide for pH adjustment.Single Dose Only – Discard unused portion. For Intravenous Use.
Recommended dosage: See prescribing information.
Store at 20°C to 25°C (68°F to 77°F), excursions permitted to 15° C to
30°C (59°F to 86° F) [see USP Controlled Room Temperature.] Protect
from light. Keep in overwrap until ready to use. Once removed from
overwrap, bag can be stored at room temperature and should be used
within 30 days.VIAFLO container is not made with natural rubber latex, DEHP, or PVC.
Rx OnlyBaxter Logo
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Made in IrelandCB-35-05-133
VIAFLO container
Symbol
07
0Symbol
Do not use
this port
Barcode
(01)00303380112203LOT
EXP
EZPE7788
NDC 0338-0108-20
250 mLNorepinephrine
Bitartrate in 5% Dextrose Injection8 mg / 250 mL
(32 mcg / mL)
For Intravenous Infusion OnlyEach 100 mL of sterile, nonpyrogenic solution contains: Norepinephrine
Bitartrate Monohydrate USP equivalent to 3.2 mg norepinephrine and
5 g Dextrose Monohydrate in Water for Injection, USP. May contain
hydrochloric acid and/or sodium hydroxide for pH adjustment.Single Dose Only – Discard unused portion. For Intravenous Use.
Recommended dosage: See prescribing information.
Store at 20°C to 25°C (68°F to 77°F), excursions permitted to 15°C to
30°C (59°F to 86°F). [See USP Controlled Room Temperature.] Protect
from light. Keep in overwrap until ready to use. Once removed from
overwrap, bag can be stored at room temperature and should be used
within 30 days.VIAFLO container is not made with natural rubber latex, DEHP, or PVC.
Rx OnlyBaxter Logo
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Made in IrelandVIAFLO container
Symbol
Do not use
this portSymbol
07
0CB-35-05-134
Symbol
1Barcode
(01)00303380108206LOT
EXP
EZPE7758
NDC 0338-0116-20
250 mLNorepinephrine
Bitartrate in 5% Dextrose Injection16 mg / 250 mL
(64 mcg / mL)
For Intravenous Infusion OnlyEach 100 mL of sterile, nonpyrogenic solution contains: Norepinephrine
Bitartrate Monohydrate USP equivalent to 6.4 mg norepinephrine and
5 g Dextrose Monohydrate in Water for Injection, USP. May contain
hydrochloric acid and/or sodium hydroxide for pH adjustment.Single Dose Only – Discard unused portion. For Intravenous Use.
Recommended dosage: See prescribing information.
Store at 20°C to 25°C (68°F to 77°F), excursions permitted to 15°C to
30°C (59°F to 86°F). [See USP Controlled Room Temperature.] Protect
from light. Keep in overwrap until ready to use. Once removed from
overwrap, bag can be stored at room temperature and should be used
within 30 days.VIAFLO container is not made with natural rubber latex, DEHP, or PVC.
Rx OnlyBaxter Logo
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Made in IrelandVIAFLO container
Symbol
Do not use
this portSymbol
07
0CB-35-05-144
Symbol
1Barcode
(01)00303380116201LOT
EXP
-
INGREDIENTS AND APPEARANCE
NOREPINEPHRINE BITARTRATE
norepinephrine bitartrate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0338-0112 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NOREPINEPHRINE BITARTRATE (UNII: IFY5PE3ZRW) (NOREPINEPHRINE - UNII:X4W3ENH1CV) NOREPINEPHRINE 4 mg in 250 mL Inactive Ingredients Ingredient Name Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) 12.5 g in 250 mL SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-0112-20 20 in 1 CARTON 01/15/2021 1 250 mL in 1 BAG; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214313 01/15/2021 NOREPINEPHRINE BITARTRATE
norepinephrine bitartrate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0338-0108 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NOREPINEPHRINE BITARTRATE (UNII: IFY5PE3ZRW) (NOREPINEPHRINE - UNII:X4W3ENH1CV) NOREPINEPHRINE 8 mg in 250 mL Inactive Ingredients Ingredient Name Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) 12.5 g in 250 mL SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-0108-20 20 in 1 CARTON 01/15/2021 1 250 mL in 1 CONTAINER; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214313 01/15/2021 NOREPINEPHRINE BITARTRATE
norepinephrine bitartrate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0338-0116 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NOREPINEPHRINE BITARTRATE (UNII: IFY5PE3ZRW) (NOREPINEPHRINE - UNII:X4W3ENH1CV) NOREPINEPHRINE 16 mg in 250 mL Inactive Ingredients Ingredient Name Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) 12.5 g in 250 mL SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-0116-20 20 in 1 CARTON 11/21/2023 1 250 mL in 1 BAG; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214313 11/21/2023 Labeler - Baxter Healthcare Corporation (005083209) Establishment Name Address ID/FEI Business Operations Baxter Healthcare S.A. 988899845 ANALYSIS(0338-0112, 0338-0108, 0338-0116) , MANUFACTURE(0338-0112, 0338-0108, 0338-0116) , LABEL(0338-0112, 0338-0108, 0338-0116) , PACK(0338-0112, 0338-0108, 0338-0116) , STERILIZE(0338-0112, 0338-0108, 0338-0116) Establishment Name Address ID/FEI Business Operations Bieffe Medital S.p.A. 437668413 ANALYSIS(0338-0112, 0338-0108, 0338-0116)