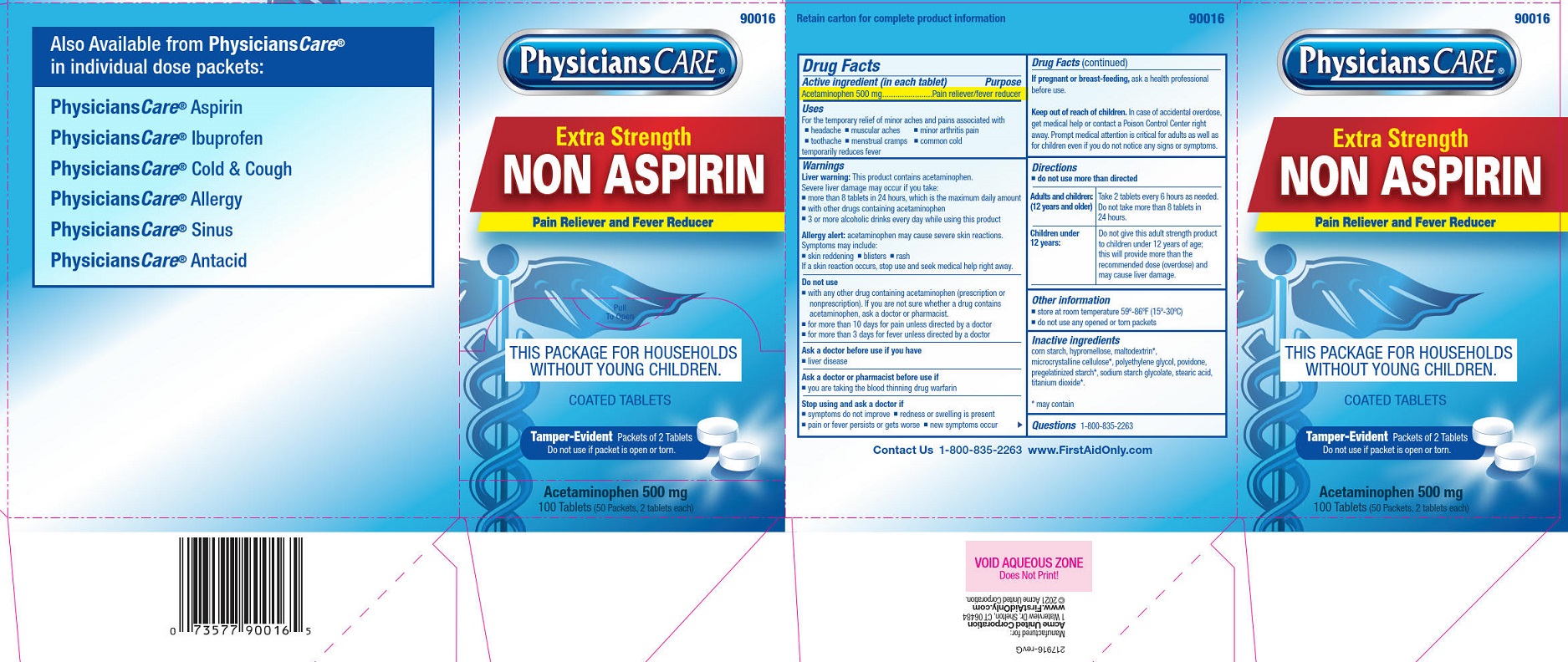
PHYSICIANSCARE NON ASPIRIN EXTRA STRENGTH- acetaminophen tablet, film coated
Acme United Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
PhysiciansCare Non Aspirin Extra Strength (Acetaminophen)
Uses
For the temporary relief of minor aches and pains associated with
■ headache
■ muscular aches
■ minor arthritis pain
■ common cold
■ toothache
■ menstrual cramps
temporarily reduces fever.
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
■ more than 8 tablets in 24 hours, which is the maximum daily amount
■ with other drugs containing acetaminophen
■ 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
■ skin reddening
■ blisters
■ rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
■ with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a
drug contains acetaminophen, ask a doctor or pharmacist.
■ for more than 10 days for pain unless directed by a doctor
■ for more than 3 days for fever unless directed by a doctor
Directions
■ do not use more than directed
Adults and children: (12 years and older)
Take 2 tablets every 6 hours as needed. Do not take more than 8 tablets in 24 hours.
Children under 12 years: Do not give this adult strength product to children under 12 years of age; this will provide more than therecommended dose (overdose) and may cause liver damage.
Other information
■ store at room temperature 59º-86ºF (15º-30ºC)
■ do not use any opened or torn packets
Inactive ingredients
corn starch, hypromellose, maltodextrin*, microcrystalline cellulose*, polyethylene glycol, povidone, pregelatinized starch*, sodium starch glycolate, stearic acid, titanium dioxide*.
* may contain
PhysiciansCare XS Non Aspirin Label
90016
Physicians Care®
Extra Strength
Non Aspirin
Pain Reliever and Fever Reducer
Pull To Open
This Package For Househoilds Without Young Children.
Coated Tablets
Tamper-Evident Packets of 2 Tablets
Do not use if oacket is open or torn.
Acetaminophen 500 mg
100 Tablets (50 Packets, 2 tablets each)
| PHYSICIANSCARE NON ASPIRIN
EXTRA STRENGTH
acetaminophen tablet, film coated |
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Acme United Corporation (001180207) |