Label: QUADRAPAX- phenobarbital, hyoscyamine sulfate, atropine sulfate, scopolamine hydrobromide elixir
-
Contains inactivated NDC Code(s)
NDC Code(s): 21695-970-16 - Packager: Rebel Distributors Corp
- This is a repackaged label.
- Source NDC Code(s): 42192-520
- Category: HUMAN PRESCRIPTION DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated September 13, 2011
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION:
Each 5 mL (one teaspoonful) for oral administration contains:
Phenobarbital, USP.......................................................16.2 mg
(WARNING: May be habit forming)
Hyoscyamine sulfate, USP.........................................0.1037 mg
Atropine sulfate, USP.................................................0.0194 mg
Scopolamine hydrobromide, USP................................0.0065 mg
Alcohol...............................................................................23%
-
INACTIVE INGREDIENTS:
Artificial grape flavor, ethyl alcohol, FD and C blue #1, FD and C red #40, glycerin, purified water USP, sodium saccharin, sorbitol solution 70%, and sucrose.
Phenobarbital is a barbiturate with the chemical name 5-Ethyl-5-phenylbarbituric acid. It has the following structural formula:
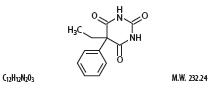
Hyoscyamine sulfate is a belladonna alkaloid with the chemical name Benzeneacetic acid, α-(hydroxmethyl)-, 8-methyl-8-azabicyclo[3.2.1]oct-3-yl ester, [3(S)-endo]-,sulfate (2:1), dihydrate. It has the following structural formula:
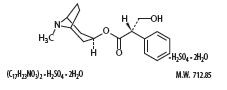
Atropine sulfate is belladonna alkaloid with the chemical name: Benzeneacetic acid, α-(hydroxymethyl)-, 8-methyl-8-azabicyclo[3.2.1]oct-3-yl ester, endo (±)-, sulfate (2:1) (salt), monohydrate. It has the following structural formula:
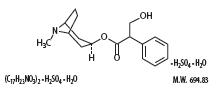
Scopolamine is a belladonna alkaloid with the chemical name Benezeneacetic acid, α-(hydroxymethyl)-, 9-methyl-3-oxa-9-azatricyclo[3.31.0.2,4]non-[7-yl ester, hydrobromide, rihydrate, [7(S)-(1α,2β,4β,5α,7β)]-. It has the following structural formula:
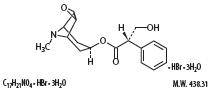
-
CLINICAL PHARMACOLOGY:
Phenobarbital is a barbiturate, nonselective central nervous system depressant which is primarily used as a sedative hypnotic and also as an anticonvulsant in subhypnotic doses. Atropine Sulfate, Hyoscyamine Sulfate and Scopolamine Hydrobromide are belladonna alkaloids classified as anticholinergic, antimuscarinic drugs and act to inhibit muscarinic actions of acetycholine at postganglionic parasympathetic neuron effector sites. These drugs are also used as antispasmodics due to their anticholinergic action and produce the effect in the body of reduced muscle spasms in the digestive or urinary tract, and reduced fluid secretions from certain glands or organs. This drug combination provides peripheral anticholinergic/antispasmodic action and mild sedation.
-
INDICATIONS AND USAGE:
FDA has classified the following indications as "possibly" effective: For use as adjunctive therapy in the treatment of irritable bowel syndrome (irritable colon, spastic colon, mucous colitis) and acute enterocolitis. May also be useful as adjunctive therapy in the treatment of duodenal ulcer.
IT HAS NOT BEEN SHOWN CONCLUSIVELY WHETHER ANTICHOLINERGIC/ANTISPASMODIC DRUGS AID IN THE HEALING OF A DUODENAL ULCER, DECREASE THE RATE OF RECURRENCES OR PREVENT COMPLICATIONS.
-
CONTRAINDICATIONS:
Quadrapax Elixir is contraindicated in patients with known hypersensitivity to any of the ingredients. Phenobarbital is contraindicated in patients with acute intermittent porphyria and in those patients in which phenobarbital produces restlessness and/or excitement.
It is also contraindicated in patients with glaucoma, obstructive uropathy; paralytic ileus; myasthenia gravis; intestinal atony; unstable cardiovascular status in acute hemorrhage; hiatal hernia associated with reflux esophagitis; obstructive disease of the gastrointestinal tract; or severe ulcerative colitis.
-
WARNINGS:
Heat prostration can occur with belladonna alkaloids in high temperatures.
Diarrhea may be an early symptom of incomplete intestinal obstruction, especially in patients with ileostomy or colostomy. In this instance, treatment with this drug could be harmful.
Quadrapax Elixir
may produce drowsiness and blurred vision. The patient should be warned about engaging in hazardous work or activities requiring mental alertness, such as operating a motor vehicle or other machinery. Phenobarbital may decrease the effect of anticoagulants, and larger doses of the anticoagulant may be needed for optimal effect. When phenobarbital is discontinued, the dose of the anticoagulant may have to be decreased. Phenobarbital may be habit forming and should not be administered to patients who are susceptible to addiction or to those with a history of physical and/or psychological drug dependence.
Barbiturates should be used with caution in patients with hepatic dysfunction.
-
PRECAUTIONS:
GENERAL:
Use with caution in patients with prostatic hypertrophy, autonomic neuropathy, hepatic or renal disease, hyperthyroidism, coronary heart disease, congestive heart failure, cardiac arrhythmias, tachycardia, and hypertension.
Belladonna alkaloids may produce a delay in gastric emptying (antral stasis) which would complicate the management of gastric ulcer. Do not rely on the use of the drug in the presence of complication of biliary tract disease. Theoretically, with overdosage, a curare-like action may occur.
INFORMATION FOR PATIENTS:
Practitioners should give the following information and instructions to patients:
- Do not increase the dose of this drug without consulting a physician.
- Do not share this medication with others.
The use of this product caries with it an associated risk of psychological and/or physical dependence.
The use of this product may impair mental and/or physical abilities required for the performance of potentially hazardous tasks such as driving or operating machinery.
Use of this product with alcohol may result in additional central nervous system depressant effects.
Tell your doctor or pharmacist if you also take antihistamines, anti-seizure drugs, medicine for sleep or anxiety, muscle relaxants, narcotic pain relievers, or psychiatric medicines.
This drug may increase the risk for heatstroke because it decreases sweating. Avoid becoming overheated in hot weather, saunas, and during exercise or other strenuous activity.
DRUG INTERACTIONS:
Do not take this medicine with the following medications:
- voriconazole
- pramlintide.
This medicine may also interact with the following medication:
CNS depressants
cyclosporine
female hormones, like estrogens or progestins and birth control pills
medicines for HIV infection like indinavir, nelfinavir, ritonavir, and saquinavir
warfarin
amantadine
anti-Parkinson's drugs levodopa, benztropine, trihexphenidyl
antispasmodic drugs clidinium, dicyclomine, propantheline
MAO inhibitors furazolidone, isocarboxazid, linezolid, moclobemide, phenelzine, procarbazine, rasagiline, selegiline, tranylcpromine
anti-arrhythmic drugs disopyramide, procainamide, quinidine
azole anti-fungal drugs ketoconazole, itraconzole
bisphosphonate drugs alendronate, risedronate
corticosteroid prednisone
digoxin (slow-dissolving tablets)
phenothiazines (Promethazine)
potassium tablets/capsules
anti-seizure drug carbamazepine
anti-anxiety drugs alprazolam, diazepam, zolpidem
narcotic pain relievers
psychiatric drugs chlorpromazine, haloperidol, amitriptyline,risperidone
certain antihistamines diphenhydramine, meclizine
muscle relaxants
CARCINOGENESIS, MUTAGENESIS, IMPAIRMENT OF FERTILITY:
Long-term studies in animals have not been performed to evaluate carcinogenic potential.
PREGNANCY TERATOGENIC EFFECTS:
Pregnancy Category D.
Quadrapax Elixir
can cause fetal harm when administered to a pregnant woman. Retrospective case-controlled studies have suggested a connection between the maternal consumption of phenobarbital and higher than expected incidence of fetal abnormalities. Following oral administration, phenobarbital readily crosses the placental barrier and is distributed throughout fetal tissues with highest concentrations found in the placenta, fetal liver, and brain. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus.
NONTERATOGENIC EFFECTS:
Reports of infants suffering from long-term phenobarbital exposure in utero included the acute withdrawal syndrome of seizures and hyperirritability from birth to a delayed onset of up to 14 days.
LABOR AND DELIVERY:
Administration of
Quadrapax Elixir
to the mother during labor may result in respiratory depression in the newborn. Premature infants are particularly susceptible to the depressant effects.
NURSING MOTHERS:
Caution should be exercised when
Quadrapax Elixir
is administered to a nursing woman. The active ingredients contained in this drug product are known to be excreted in human milk.
SPECIAL PATIENT POPULATION:
Dosage should be reduces in the debilitated because these patients may be more sensitive to phenobarbital. Dosage should be reduced for patients with impaired renal function or hepatic disease.
PEDIATRIC USE:
Use
Quadrapax Elixir
with caution in children as they may be more susceptible to the effects of this product especially infants, children with Down's syndrome, spastic paralysis or brain damage.
GERIATRIC USE:
Sedating drugs may cause confusion and over-sedation in the elderly; elderly patients generally should be started on low doses of
Quadrapax Elixir
and observed closely. This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
-
ADVERSE REACTIONS:
Adverse reactions associated with anticholinergics and/or anticonvulsants are: dry mouth; tachycardia; urinary hesitancy and retention; palpitation; blurred vision; prolonged pupil dilation; cycloplegia; increased ocular tension; loss of taste sense; headache; nervousness; drowsiness; weakness; dizziness; insomnia; nausea; vomiting; severe allergic reaction or drug idiosyncrasies, including anaphylaxis, hives and/or other dermal manifestations; decreased sweating; impotence; suppression of lactation; constipation; bloated feeling and musculoskeletal pain. Elderly patients may react with symptoms of excitement, agitation and drowsiness to even small doses of the drug. Phenobarbital may produce excitement in some patients, rather than a sedative effect. In patients habituated to barbiturates, abrupt withdrawal may produce delirium or convulsions.
-
DRUG ABUSE AND DEPENDENCE:
This product is subject to the provisions of the Controlled Substance Act and has been placed in Schedule IV. Phenobarbital may be habit-forming. Tolerance, psychological dependence, and physical dependence may occur following prolonged use.
Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
-
OVERDOSAGE
An overdose of
Quadrapax Elixir
is a potentially lethal overdose situation. Call your doctor or a Poison Control Center immediately if an overdose is suspected.
SIGNS AND SYMPTOMS:
Characteristics of phenobarbital overdose include decreased consciousness leading to coma, bradycardia, bradypnea, hypothermia and hypotention. Overdose may also lead to pulmonary edema and acute renal failure as result of shock. Other symptoms of an overdose with this product include headache, nausea, vomiting, tachycardia, severe or persistent blurred vision, dilated pupils, hot and dry skin, dizziness or disorientation, severe dryness of the mouth, difficulty in swallowing, and CNS stimulation.
RECOMMENDED TREATMENT:
Treatment of overdose is supportive, and consists mainly in the maintenance of airway patency through endotracheal intubation and mechanical ventilation, correction of bradycardia and hypotention with intravenous fluids and vasopressors, if necessary, and removal of as much drug as possible from the body. Depending on how much time has elapsed since ingestion of the drug, this may be accomplished through gastric lavage or use of activated charcoal.
-
DOSAGE AND ADMINISTRATION:
The dosage of
Quadrapax Elixir
should be adjusted to the needs of the individual patient to assure symptomatic control with a minimum of adverse effects.
Adults:
One or two teaspoonfuls of elixir three to four times a day according to conditions and severity of symptoms.
Pediatric patients:
may be dosed every 4 to 6 hours.
Starting dosage
Body weight q4h q6h 10 lb. (4.5 kg)
0.5 mL
0.75 mL
20 lb. (9.1 kg)
1.0 mL
1.5 mL
30 lb. (13.6 kg)
1.5 mL
2.0 mL
50 lb. (22.7 kg)
1/2 tsp
3/4 tsp
75 lb. (34 kg)
3/4 tsp
1 tsp
100 lb. (45.4 kg)
1 tsp
1 1/2 tsp
Children under 2 years of age:
as directed by a physician.
-
HOW SUPPLIED:
Quadrapax Elixir is a purple colored, grape flavored liquid.
1 Pint (16 fl oz.) bottles NDC 21695-970-16.
Store at 20°- 25°C(68°- 77°F) [see USP Controlled Room Temperature].
Excursions permitted to 15°- 30°C(59°- 86°F).
Tamper evident by foil seal under cap. Do not use if foil seal is broken or missing.
WARNINGS: KEEP THIS AND ALL DRUGS OUT OF THE REACH OF CHILDREN. IN CASE OF ACCIDENTAL OVERDOSE, SEEK PROFESSIONAL ASSISTANCE OR CONTACT A POISON CONTROL CENTER IMMEDIATELY.
Manufactured for:
Acella PHARMACEUTICALS, LLC
Acella Pharmaceuticals, LLC
Alpharetta, GA 30009
Iss. 03/11
Relabeled by:
Rebel Distributors Corp
Thousand Oaks, CA 91320
-
PRODUCT PACKAGING:
The packaging below represents the labeling currently used:
Principal display panel and side panel for 473 mL label:
NDC 21695-970-16
QUADRAPAX ELIXIRGrape Flavored
Each 5 mL (one teaspoonful) for oral administration contains:
Phenobarbital, USP.................................................16.2 mg
(WARNING: may be habit forming)
Hyoscyamine sulfate, USP...................................0.1037 mg
Atropine sulfate, USP...........................................0.0194 mg
Scopolamine hydrobromide, USP..........................0.0065 mg
Alcohol........................................................................23%
CIV
Bulk Container
Not For Household Use
Rx ONLY
1 Pint
(16 fl oz / 473 mL)
Acella PHARMACEUTICALS, LLC
Tamper evident by foil seal under cap. Do not use if foil seal is broken or missing.
THIS BOTTLE IS NOT TO BE DISPENSED TO THE CONSUMER.
WARNINGS: KEEP THIS AND ALL DRUGS OUT OF THE REACH OF CHILDREN. IN CASE OF ACCIDENTAL OVERDOSE,
SEEK PROFESSIONAL ASSISTANCE OR CONTACT A POISON CONTROL CENTER IMMEDIATELY.May be habit forming.
Store at 20°- 25°C (68°- 77°F) [see USP Controlled Room Temperature].
Excursions permitted to 15°- 30°C (59°- 86°F).
Avoid freezing.
Protect from light.
Dispense in a tight, light-resistant cap.
Manufactured for:
Acella Pharmaceuticals, LLC
Alpharetta, GA 30009
Iss. 03/11
Relabeled by:
Rebel Distributors Corp
Thousand Oaks, CA 91320

-
INGREDIENTS AND APPEARANCE
QUADRAPAX
phenobarbital, hyoscyamine sulfate, atropine sulfate, scopolamine hydrobromide elixirProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:21695-970(NDC:42192-520) Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Phenobarbital (UNII: YQE403BP4D) (Phenobarbital - UNII:YQE403BP4D) Phenobarbital 16.2 mg in 5 mL Hyoscyamine Sulfate (UNII: F2R8V82B84) (Hyoscyamine - UNII:PX44XO846X) Hyoscyamine Sulfate 0.1037 mg in 5 mL Atropine Sulfate (UNII: 03J5ZE7KA5) (Atropine - UNII:7C0697DR9I) Atropine Sulfate 0.0194 mg in 5 mL Scopolamine Hydrobromide (UNII: 451IFR0GXB) (Scopolamine - UNII:DL48G20X8X) Scopolamine Hydrobromide 0.0065 mg in 5 mL Inactive Ingredients Ingredient Name Strength Alcohol (UNII: 3K9958V90M) Glycerin (UNII: PDC6A3C0OX) Water (UNII: 059QF0KO0R) Saccharin Sodium (UNII: SB8ZUX40TY) Sorbitol (UNII: 506T60A25R) Sucrose (UNII: C151H8M554) Product Characteristics Color Score Shape Size Flavor GRAPE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21695-970-16 473 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug other 05/06/2011 Labeler - Rebel Distributors Corp (118802834) Establishment Name Address ID/FEI Business Operations Rebel Distributors Corp 118802834 RELABEL, REPACK

