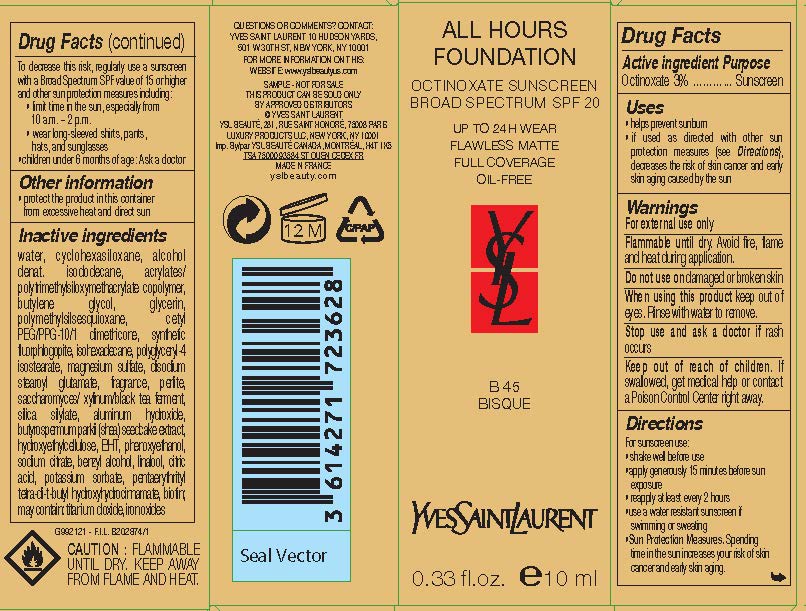
Label: YVES SAINT LAURENT ALL HOURS FOUNDATION BROAD SPECTRUM SPF 20 SUNSCREEN- octinoxate lotion
-
NDC Code(s):
49967-362-01,
49967-362-02,
49967-362-03,
49967-362-04, view more49967-362-05, 49967-362-06, 49967-362-07
- Packager: L'Oreal USA Products Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 24, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Flammable until dry.
- Do not use
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
-
Directions
For sunscreen use:
- shake well before use
- apply liberally 15 minutes before sun exposure
- reapply at least every 2 hours
- use a water resistant sunscreen if swimming or sweating
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- children under 6 months of age: Ask a doctor
- Other information
-
Inactive ingredients
water, cyclopentasiloxane, alcohol denat., isododecane, acrylates/polytrimethylsiloxymethacrylate copolymer, butylene glycol, glycerin, polymethylsilsesquioxane, cetyl PEG/PPG-10/1 dimethicone, synthetic fluorphlogopite, isohexadecane, polyglyceryl-4 isostearate, magnesium sulfate, disodium stearoyl glutamate, fragrance, perlite, saccharomyces/xylinum/black tea ferment, silica silylate, aluminum hydroxide, butyrospermum parkii (shea) seedcake extract, hydroxyethylcellulose, BHT, phenoxyethanol, sodium citrate, benzyl alcohol, linalool, citric acid, potassium sorbate, pentaerythrityl tetra-di-t-butyl hydroxyhydrocinnamate, biotin; may contain: titanium dioxide, iron oxides
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
YVES SAINT LAURENT ALL HOURS FOUNDATION BROAD SPECTRUM SPF 20 SUNSCREEN
octinoxate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49967-362 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) ALCOHOL (UNII: 3K9958V90M) ISODODECANE (UNII: A8289P68Y2) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERIN (UNII: PDC6A3C0OX) POLYMETHYLSILSESQUIOXANE (11 MICRONS) (UNII: Z570VEV8XK) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) ISOHEXADECANE (UNII: 918X1OUF1E) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) PERLITE (UNII: 0SG101ZGK9) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) HYDROXYETHYL CELLULOSE (100 MPA.S AT 2%) (UNII: R33S7TK2EP) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) PHENOXYETHANOL (UNII: HIE492ZZ3T) SODIUM CITRATE (UNII: 1Q73Q2JULR) BENZYL ALCOHOL (UNII: LKG8494WBH) LINALOOL, (+/-)- (UNII: D81QY6I88E) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) BIOTIN (UNII: 6SO6U10H04) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49967-362-01 1 in 1 CARTON 06/01/2017 1 25 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:49967-362-02 125 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/01/2017 12/30/2024 3 NDC:49967-362-03 1 in 1 CARTON 06/01/2017 12/30/2024 3 10 mL in 1 BOTTLE; Type 0: Not a Combination Product 4 NDC:49967-362-04 1 in 1 CARTON 06/01/2017 12/30/2024 4 5 mL in 1 BOTTLE; Type 0: Not a Combination Product 5 NDC:49967-362-05 1 mL in 1 PACKET; Type 0: Not a Combination Product 06/01/2017 12/30/2024 6 NDC:49967-362-06 1.2 mL in 1 BLISTER PACK; Type 0: Not a Combination Product 02/08/2019 12/30/2024 7 NDC:49967-362-07 1 in 1 CARTON 07/30/2020 12/30/2024 7 8 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 06/01/2017 Labeler - L'Oreal USA Products Inc (002136794) Establishment Name Address ID/FEI Business Operations SICOS ET CIE 276993581 manufacture(49967-362) , pack(49967-362)