DEXMETHYLPHENIDATE HYDROCHLORIDE- dexmethylphenidate hydrochloride tablet
Avera McKennan Hospital
----------
Dexmethylphenidate Hydrochloride Tablets CII
Rx Only
Prescribing Information
DESCRIPTION
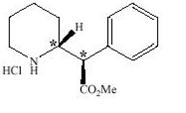
Dexmethylphenidate hydrochloride is the d- threo-enantiomer of racemic methylphenidate hydrochloride, which is a 50/50 mixture of the d-threo and l-threo-enantiomers. Dexmethylphenidate hydrochloride is a central nervous system (CNS) stimulant, available in three tablet strengths. Each tablet contains dexmethylphenidate hydrochloride 2.5, 5, or 10 mg for oral administration. Dexmethylphenidate hydrochloride is methyl α-phenyl-2-piperidineacetate hydrochloride, (R,R’)-(+)-. Its empirical formula is C 14H 19NO 2•HCl. Its molecular weight is 269.77 and its structural formula is
Note: * = asymmetric carbon centers
Dexmethylphenidate hydrochloride is a white to off white powder. Its solutions are acid to litmus. It is freely soluble in water and in methanol, soluble in alcohol, and slightly soluble in chloroform and in acetone.
Dexmethylphenidate hydrochloride tablets also contain the following inert ingredients: lactose monohydrate, sodium starch glycolate, talc, magnesium stearate, and FD&C Blue No.1 aluminum lake (2.5 mg tablet), D&C Yellow No. 10 aluminum lake (5 mg tablet); the 10 mg tablet contains no dye.
CLINICAL PHARMACOLOGY
Pharmacodynamics
Dexmethylphenidate hydrochloride is a central nervous system stimulant. Dexmethylphenidate hydrochloride, the more pharmacologically active enantiomer of the d- and l-enantiomers, is thought to block the reuptake of norepinephrine and dopamine into the presynaptic neuron and increase the release of these monoamines into the extraneuronal space. The mode of therapeutic action in Attention Deficit Hyperactivity Disorder (ADHD) is not known.
Effects on QT Interval
The effect of dexmethylphenidate hydrochloride extended-release capsules on the QT interval was evaluated in a double-blind, placebo- and open label active (moxifloxacin)-controlled study following single doses of dexmethylphenidate hydrochloride extended-release capsules 40mg in 75 healthy volunteers. ECGs were collected up to 12 hours postdose. Frederica’s method for heart rate correction was employed to derive the corrected QT interval (QTcF). The maximum mean prolongation of QTcF intervals was <5 ms, and the upper limit of the 90% confidence interval was below 10 ms for all time matched comparisons versus placebo. This was below the threshold of clinical concern and there was no evident-exposure response relationship.
Pharmacokinetics
Absorption
Dexmethylphenidate hydrochloride is readily absorbed following oral administration of dexmethylphenidate hydrochloride tablets. In patients with ADHD, plasma dexmethylphenidate concentrations increase rapidly, reaching a maximum in the fasted state at about 1 to 1.5 hours postdose. No differences in the pharmacokinetics of dexmethylphenidate hydrochloride tablets were noted following single and repeated twice daily dosing, thus indicating no significant drug accumulation in children with ADHD.
When given to children as capsules in single doses of 2.5 mg, 5 mg, and 10 mg, C
max and AUC
0-inf of dexmethylphenidate were proportional to dose. In the same study, plasma dexmethylphenidate levels were comparable to those achieved following single
dl-threo-methylphenidate HCl doses given as capsules in twice the total mg amount (equimolar with respect to dexmethylphenidate hydrochloride tablets).
Food Effects
In a single dose study conducted in adults, coadministration of 2 x 10 mg dexmethylphenidate hydrochloride tablets with a high fat breakfast resulted in a dexmethylphenidate t
max of 2.9 hours postdose as compared to 1.5 hours postdose when given in a fasting state. C
max and AUC
0-inf were comparable in both the fasted and nonfasted states.
Distribution
Plasma dexmethylphenidate concentrations in children decline exponentially following oral administration of dexmethylphenidate hydrochloride tablets.
Metabolism and Excretion
In humans, dexmethylphenidate is metabolized primarily to
d-α-phenyl-piperidine acetic acid (also known as
d-ritalinic acid) by de-esterification. This metabolite has little or no pharmacological activity. There is little or no
in vivo interconversion to the
l-threo-enantiomer, based on a finding of minute levels of
l-threo-methylphenidate being detectable in a few samples in only 2 of 58 children and adults. After oral dosing of radiolabeled racemic methylphenidate in humans, about 90% of the radioactivity was recovered in urine. The main urinary metabolite was ritalinic acid, accountable for approximately 80% of the dose.
In vitro studies showed that dexmethylphenidate did not inhibit cytochrome P450 isoenzymes.
The mean plasma elimination half-life of dexmethylphenidate is approximately 2.2 hours.
Special Populations
Gender
Pharmacokinetic parameters were similar for boys and girls (mean age 10 years).
In a single dose study conducted in adults, the mean dexmethylphenidate AUC
0-inf values (adjusted for body weight) following single 2 x 10 mg doses of dexmethylphenidate hydrochloride tablets were 25%-35% higher in adult female volunteers (n=6) compared to male volunteers (n=9). Both t
max and t
1/2 were comparable for males and females.
Race
There is insufficient experience with the use of dexmethylphenidate hydrochloride tablets to detect ethnic variations in pharmacokinetics.
Age
The pharmacokinetics of dexmethylphenidate after dexmethylphenidate hydrochloride tablets administration have not been studied in children less than 6 years of age. When single doses of dexmethylphenidate hydrochloride tablets were given to children between the ages of 6 to 12 years and healthy adult volunteers, C
max of dexmethylphenidate was similar, however, children showed somewhat lower AUCs compared to the adults.
Renal Insufficiency
There is no experience with the use of dexmethylphenidate hydrochloride tablets in patients with renal insufficiency. After oral administration of radiolabeled racemic methylphenidate in humans, methylphenidate was extensively metabolized and approximately 80% of the radioactivity was excreted in the urine in the form of ritalinic acid. Since very little unchanged drug is excreted in the urine, renal insufficiency is expected to have little effect on the pharmacokinetics of dexmethylphenidate hydrochloride tablets.
Hepatic Insufficiency
There is no experience with the use of dexmethylphenidate hydrochloride tablets in patients with hepatic insufficiency (see
PRECAUTIONS,
Drug Interactions).
Clinical Studies
Dexmethylphenidate hydrochloride tablets were evaluated in 2 double-blind, parallel-group, placebo-controlled trials in untreated or previously treated patients aged 6 to 17 years old with a DSM-IV diagnosis of Attention Deficit Hyperactivity Disorder (ADHD). Both studies included all 3 subtypes of ADHD,
i.e., Combined Type, Predominantly Inattentive Type, or Predominantly Hyperactive-Impulsive Type. While both children and adolescents were included, the sample was predominantly children, thus, the findings are most pertinent to this age group. In both studies, the primary comparison of interest was dexmethylphenidate hydrochloride tablet
versus placebo.
Dexmethylphenidate hydrochloride tablets (5, 10, or 20 mg/day total dose),
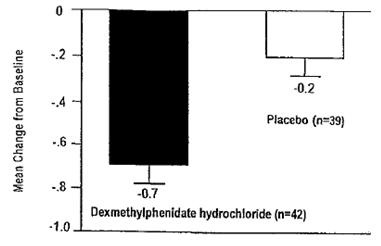
dl-threo-methylphenidate HCl (10, 20, or 40 mg/day total dose), and placebo were compared in a multicenter, 4-week, parallel group study in n=132 patients. Patients took the study medication twice daily, 3.5 to 5.5 hours between doses. Treatment was initiated with the lowest dose, and doses could be doubled at weekly intervals, depending on clinical response and tolerability, up to the maximum dose. The change from baseline to week 4 of the averaged score (an average of 2
ratings during the week) of the teacher’s version of the SNAP-ADHD Rating Scale, a scale for assessing ADHD symptoms, was the primary outcome. Patients treated with dexmethylphenidate hydrochloride tablets showed a statistically significant improvement in symptom scores from baseline over patients who received placebo.
Figure 1
Mean Change from Baseline in Teacher SNAP-ADHD Scores in a 4-week Double-Blind Placebo-Controlled Study of Dexmethylphenidate Hydrochloride Tablets*
*Figure 1: Error bars represent the standard error of the mean.
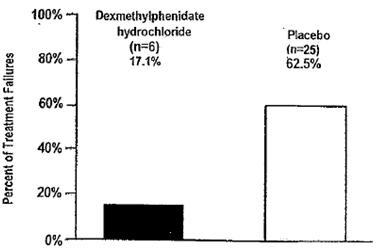
The other study, involving n=75 patients, was a multicenter, placebo-controlled, double-blind, 2-week treatment withdrawal study in children who were responders during a 6-week, open label initial treatment period. Children took study medication twice a day separated by a 3.5 to 5.5 hour interval. The primary outcome was proportion of treatment failures at the end of the 2-week withdrawal phase, where treatment failure was defined as a rating of 6 (much worse) or 7 (very much worse) on the Investigator Clinical Global Impression - Improvement (CGI-I). Patients continued on dexmethylphenidate hydrochloride tablets showed a statistically significant lower rate of failure over patients who received placebo.
Figure 2
Percent of Treatment Failures Following a 2-week Double-Blind Placebo-Controlled Withdrawal of Dexmethylphenidate Hydrochloride Tablets
INDICATION AND USAGE
Dexmethylphenidate hydrochloride tablets are indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD).
The efficacy of dexmethylphenidate hydrochloride tablets in the treatment of ADHD was established in two controlled trials of patients aged 6 to 17 years of age who met DSM-IV criteria for ADHD (see
Clinical Studies).
A diagnosis of ADHD (DSM-IV) implies the presence of hyperactive-impulsive or inattentive symptoms that cause impairment and were present before age 7 years. The symptoms must cause clinically significant impairment,
e.g., in social, academic, or occupational functioning; and be present in two or more settings,
e.g., school (or work) and at home. The symptoms must not be better accounted for by another mental disorder. For the inattentive type, at least six of the following symptoms must have persisted for at least 6 months: lack of attention to details/careless mistakes; lack of sustained attention; poor listener; failure to follow through on tasks; poor organization; avoids tasks requiring sustained mental effort; loses things; easily distracted; forgetful. For the Hyperactive-Impulsive Type, at least 6 of the following symptoms must have persisted for at least 6 months: fidgeting/squirming; leaving seat; inappropriate running/climbing; difficulty with quiet activities; “on the go,” excessive talking; blurting answers; can’t wait turn; intrusive. The Combined Type requires both inattentive and hyperactive-impulsive criteria to be met.
Special Diagnostic Considerations
Specific etiology of this syndrome is unknown, and there is no single diagnostic test. Adequate diagnosis requires the use not only of medical but of special psychological, educational, and social resources. Learning may or may not be impaired. The diagnosis must be based upon a complete history and evaluation of the child and not solely on the presence of the required number of DSM-IV characteristics.
Need for Comprehensive Treatment Program
Dexmethylphenidate hydrochloride tablets are indicated as an integral part of a total treatment program for ADHD that may include other measures (psychological, educational, social) for patients with this syndrome. Drug treatment may not be indicated for all patients with this syndrome. Stimulants are not intended for use in the patient who exhibits symptoms secondary to environmental factors and/or other primary psychiatric disorders, including psychosis. Appropriate educational placement is essential and psychosocial intervention is often helpful. When remedial measures alone are insufficient, the decision to prescribe stimulant medication will depend upon the physician’s assessment of the chronicity and severity of the patient’s symptoms.
Long-term Use
The effectiveness of dexmethylphenidate hydrochloride tablets for long-term use, i.e., for more than 6 weeks, has not been systematically evaluated in controlled trials. Therefore, the physician who elects to use dexmethylphenidate hydrochloride tablets for extended periods should periodically re-evaluate the long-term usefulness of the drug for the individual patient (see DOSAGE AND ADMINISTRATION).
CONTRAINDICATIONS
Agitation
Dexmethylphenidate hydrochloride tablets are contraindicated in patients with marked anxiety, tension, and agitation, since the drug may aggravate these symptoms.
Hypersensitivity to Methylphenidate
Dexmethylphenidate hydrochloride tablets are contraindicated in patients known to be hypersensitive to methylphenidate or other components of the product. Hypersensitivity reactions, including angioedema and anaphylactic reactions, have been observed in patients treated with methylphenidate (see ADVERSE REACTIONS).
Glaucoma
Dexmethylphenidate hydrochloride tablets are contraindicated in patients with glaucoma.
Tics
Dexmethylphenidate hydrochloride tablets are contraindicated in patients with motor tics or with a family history or diagnosis of Tourette’s syndrome (see
ADVERSE REACTIONS).
Monoamine Oxidase Inhibitors
Dexmethylphenidate hydrochloride tablets are contraindicated during treatment with monoamine oxidase inhibitors, and also within a minimum of 14 days following discontinuation of a monoamine oxidase inhibitor (hypertensive crises may result).
WARNINGS
Serious Cardiovascular Events
Sudden Death and Preexisting Structural Cardiac Abnormalities or Other Serious Heart Problems
Children and Adolescents
Sudden death has been reported in association with CNS stimulant treatment at usual doses in children and adolescents with structural cardiac abnormalities or other serious heart problems. Although some serious heart problems alone carry an increased risk of sudden death, stimulant products generally should not be used in children or adolescents with known serious structural cardiac abnormalities, cardiomyopathy, serious heart rhythm abnormalities, or other serious cardiac problems that may place them at increased vulnerability to the sympathomimetic effects of a stimulant drug.
Adults
Sudden death, stroke, and myocardial infarction have been reported in adults taking stimulant drugs at usual doses for ADHD. Although the role of stimulants in these adult cases is also unknown, adults have a greater likelihood than children of having serious structural cardiac abnormalities, cardiomyopathy, serious heart rhythm abnormalities, coronary artery disease, or other serious cardiac problems. Adults with such abnormalities should also generally not be treated with stimulant drugs.
Hypertension and Other Cardiovascular Conditions
Stimulant medications cause a modest increase in average blood pressure (about 2-4 mmHg) and average heart rate (about 3-6 bpm), and individuals may have larger increases. While the mean changes alone would not be expected to have short-term consequences, all patients should be monitored for larger changes in heart rate and blood pressure. Caution is indicated in treating patients whose underlying medical conditions might be compromised by increases in blood pressure or heart rate, e.g., those with preexisting hypertension, heart failure, recent myocardial infarction, or ventricular arrhythmia.
Assessing Cardiovascular Status in Patients being Treated with Stimulant Medications
Children, adolescents, or adults who are being considered for treatment with stimulant medications should have a careful history (including assessment for a family history of sudden death or ventricular arrhythmia) and physical exam to assess for the presence of cardiac disease, and should receive further cardiac evaluation if findings suggest such disease (e.g., electrocardiogram and echocardiogram). Patients who develop symptoms such as exertional chest pain, unexplained syncope, or other symptoms suggestive of cardiac disease during stimulant treatment should undergo a prompt cardiac evaluation.
Psychiatric Adverse Events
Preexisting Psychosis
Administration of stimulants may exacerbate symptoms of behavior disturbance and thought disorder in patients with a preexisting psychotic disorder.
Bipolar Illness
Particular care should be taken in using stimulants to treat ADHD in patients with comorbid bipolar disorder because of concern for possible induction of a mixed/manic episode in such patients. Prior to initiating treatment with a stimulant, patients with comorbid depressive symptoms should be adequately screened to determine if they are at risk for bipolar disorder; such screening should include a detailed psychiatric history, including a family history of suicide, bipolar disorder, and depression.
Emergence of New Psychotic or Manic Symptoms
Treatment emergent psychotic or manic symptoms, e.g., hallucinations, delusional thinking, or mania in children and adolescents without a prior history of psychotic illness or mania can be caused by stimulants at usual doses. If such symptoms occur, consideration should be given to a possible causal role of the stimulant, and discontinuation of treatment may be appropriate. In a pooled analysis of multiple short-term, placebo-controlled studies, such symptoms occurred in about 0.1% (4 patients with events out of 3,482 exposed to methylphenidate or amphetamine for several weeks at usual doses) of stimulant-treated patients compared to 0 in placebo-treated patients.
Aggression
Aggressive behavior or hostility is often observed in children and adolescents with ADHD, and has been reported in clinical trials and the postmarketing experience of some medications indicated for the treatment of ADHD. Although there is no systematic evidence that stimulants cause aggressive behavior or hostility, patients beginning treatment for ADHD should be monitored for the appearance of or worsening of aggressive behavior or hostility.
Long-Term Suppression of Growth
Careful follow-up of weight and height in children ages 7 to 10 years who were randomized to either methylphenidate or nonmedication treatment groups over 14 months, as well as in naturalistic subgroups of newly methylphenidate-treated and nonmedication treated children over 36 months (to the ages of 10 to 13 years), suggests that consistently medicated children (i.e., treatment for 7 days per week throughout the year) have a temporary slowing in growth rate (on average, a total of about 2 cm less growth in height and 2.7 kg less growth in weight over 3 years), without evidence of growth rebound during this period of development. Published data are inadequate to determine whether chronic use of amphetamines may cause a similar suppression of growth, however, it is anticipated that they likely have this effect as well. Therefore, growth should be monitored during treatment with stimulants, and patients who are not growing or gaining height or weight as expected may need to have their treatment interrupted.
Seizures
There is some clinical evidence that stimulants may lower the convulsive threshold in patients with prior history of seizures, in patients with prior EEG abnormalities in absence of seizures, and, very rarely, in patients without a history of seizures and no prior EEG evidence of seizures. In the presence of seizures, the drug should be discontinued.
Priapism
Prolonged and painful erections, sometimes requiring surgical intervention, have been reported with methylphenidate products in both pediatric and adult patients. Priapism was not reported with drug initiation but developed after some time on the drug, often subsequent to an increase in dose. Priapism has also appeared during a period of drug withdrawal (drug holidays or discontinuation). Patients who develop abnormally sustained or frequent and painful erections should seek immediate medical attention.
Peripheral Vasculopathy, Including Raynaud’s Phenomenon
Stimulants, including dexmethylphenidate hydrochloride tablets, used to treat ADHD are associated with peripheral vasculopathy, including Raynaud’s phenomenon. Signs and symptoms are usually intermittent and mild; however, very rare sequelae include digital ulceration and/or soft tissue breakdown. Effects of peripheral vasculopathy, including Raynaud’s phenomenon, were observed in postmarketing reports at different times and at therapeutic doses in all age groups throughout the course of treatment. Signs and symptoms generally improve after reduction in dose or discontinuation of drug. Careful observation for digital changes is necessary during treatment with ADHD stimulants. Further clinical evaluation (e.g., rheumatology referral) may be appropriate for certain patients.
Visual Disturbance
Difficulties with accommodation and blurring of vision have been reported with stimulant treatment.
Use in Children Under 6 Years of Age
Dexmethylphenidate hydrochloride tablets should not be used in children under 6 years, since safety and efficacy in this age group have not been established.
DRUG DEPENDENCE:
Dexmethylphenidate hydrochloride tablets should be given cautiously to patients with a history of drug dependence or alcoholism. Chronic, abusive use can lead to marked tolerance and psychological dependence with varying degrees of abnormal behavior. Frank psychotic episodes can occur, especially with parenteral abuse. Careful supervision is required during drug withdrawal from abusive use since severe depression may occur. Withdrawal following chronic therapeutic use may unmask symptoms of the underlying disorder that may require follow-up.
PRECAUTIONS
Hematologic Monitoring
Periodic CBC, differential, and platelet counts are advised during prolonged therapy.
Information for Patients
Prescribers or other health professionals should inform patients, their families, and their caregivers about the benefits and risks associated with treatment with dexmethylphenidate and should counsel them in its appropriate use. A patient Medication Guide is available for dexmethylphenidate hydrochloride tablets. The prescriber or health professional should instruct patients, their families, and their caregivers to read the Medication Guide and should assist them in understanding its contents. Patients should be given the opportunity to discuss the contents of the Medication Guide and to obtain answers to any questions they may have. The complete text of the Medication Guide is reprinted at the end of this document.
Priapism
Advise patients, caregivers, and family members of the possibility of painful or prolonged penile erections (priapism).
Instruct the patient to seek immediate medical attention in the event of priapism.
Circulation problems in fingers and toes [Peripheral vasculopathy, including Raynaud’s phenomenon]:
- Instruct patients beginning treatment with dexmethylphenidate hydrochloride tablets about the risk of peripheral vasculopathy, including Raynaud’s Phenomenon, and in associated signs and symptoms: fingers or toes may feel numb, cool, painful, and/or may change color from pale, to blue, to red.
- Instruct patients to report to their physician any new numbness, pain, skin color change, or sensitivity to temperature in fingers or toes.
- Instruct patients to call their physician immediately with any signs of unexplained wounds appearing on fingers or toes while taking dexmethylphenidate hydrochloride tablets.
- Further clinical evaluation (e.g., rheumatology referral) may be appropriate for certain patients.
Drug Interactions
Methylphenidate may decrease the effectiveness of drugs used to treat hypertension. Because of possible effects on blood pressure, dexmethylphenidate hydrochloride tablets should be used cautiously with pressor agents.
Human pharmacologic studies have shown that racemic methylphenidate may inhibit the metabolism of coumarin anticoagulants, anticonvulsants (
e.g., phenobarbital, phenytoin, primidone), and some antidepressants (tricyclics and selective serotonin reuptake inhibitors). Downward dose adjustments of these drugs may be required when given concomitantly with methylphenidate. It may be necessary to adjust the dosage and monitor plasma drug concentration (or, in the case of coumarin, coagulation times), when initiating or discontinuing concomitant methylphenidate.
Carcinogenesis, Mutagenesis, and Impairment of Fertility
Lifetime carcinogenicity studies have not been carried out with dexmethylphenidate. In a lifetime carcinogenicity study carried out in B6C3F1 mice, racemic methylphenidate caused an increase in hepatocellular adenomas, and in males only, an increase in hepatoblastomas at a daily dose of approximately 60 mg/kg/day. Hepatoblastoma is a relatively rare rodent malignant tumor type. There was no increase in total malignant hepatic tumors. The mouse strain used is sensitive to the development of hepatic tumors, and the significance of these results to humans is unknown.
Racemic methylphenidate did not cause any increase in tumors in a lifetime carcinogenicity study carried out in F344 rats; the highest dose used was approximately 45 mg/kg/day.
In a 24-week study of racemic methylphenidate in the transgenic mouse strain p53+/-, which is sensitive to genotoxic carcinogens, there was no evidence of carcinogenicity. Mice were fed diets containing the same concentrations as in the lifetime carcinogenicity study; the high-dose group was exposed to 60-74 mg/kg/day of racemic methylphenidate.
Dexmethylphenidate was not mutagenic in the
in vitro Ames reverse mutation assay, the
in vitro mouse lymphoma cell forward mutation assay, or the
in vivo mouse bone marrow micronucleus test.
Racemic methylphenidate was not mutagenic in the
in vitro Ames reverse mutation assay or the
in vitro mouse lymphoma cell forward mutation assay, and was negative
in vivo in the mouse bone marrow micronucleus assay. However, sister chromatid exchanges and chromosome aberrations were increased, indicative of a weak clastogenic response, in an
in vitro assay of racemic methylphenidate in cultured Chinese Hamster Ovary (CHO) cells.
Racemic methylphenidate did not impair fertility in male or female mice that were fed diets containing the drug in an 18-week Continuous Breeding study. The study was conducted at doses of up to 160 mg/kg/day.
Pregnancy
Pregnancy Category C
In studies conducted in rats and rabbits, dexmethylphenidate was administered orally at doses of up to 20 and 100 mg/kg/day, respectively, during the period of organogenesis. No evidence of teratogenic activity was found in either the rat or rabbit study; however, delayed fetal skeletal ossification was observed at the highest dose level in rats. When dexmethylphenidate was administered to rats throughout pregnancy and lactation at doses of up to 20 mg/kg/day, postweaning body weight gain was decreased in male offspring at the highest dose, but no other effects on postnatal development were observed. At the highest doses tested, plasma levels (AUCs) of dexmethylphenidate in pregnant rats and rabbits were approximately 5 and 1 times, respectively, those in adults dosed with the maximum recommended human dose of 20 mg/day.
Racemic methylphenidate has been shown to have teratogenic effects in rabbits when given in doses of 200 mg/kg/day throughout organogenesis.
Adequate and well-controlled studies in pregnant women have not been conducted. Dexmethylphenidate hydrochloride tablets should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
It is not known whether dexmethylphenidate is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised if dexmethylphenidate hydrochloride tablets are administered to a nursing woman.
Pediatric Use
The safety and efficacy of dexmethylphenidate hydrochloride tablets in children under 6 years old have not been established. Long-term effects of dexmethylphenidate hydrochloride tablets in children have not been well established (see
WARNINGS).
In a study conducted in young rats, racemic methylphenidate was administered orally at doses of up to 100 mg/kg/day for 9 weeks, starting early in the postnatal period (Postnatal Day 7) and continuing through sexual maturity (Postnatal Week 10). When these animals were tested as adults (Postnatal Weeks 13-14), decreased spontaneous locomotor activity was observed in males and females previously treated with 50 mg/kg/day (approximately 6 times the maximum recommended human dose [MRHD] of racemic methylphenidate on a mg/m
2 basis) or greater, and a deficit in the acquisition of a specific learning task was seen in females exposed to the highest dose (12 times the racemic MRHD on a mg/m
2 basis). The no effect level for juvenile neurobehavioral development in rats was 5 mg/kg/day (half the racemic MRHD on a mg/m
2 basis). The clinical significance of the long-term behavioral effects observed in rats is unknown.
ADVERSE REACTIONS
The premarketing development program for dexmethylphenidate hydrochloride tablets included exposures in a total of 696 participants in clinical trials (684 patients, 12 healthy adult subjects). These participants received dexmethylphenidate hydrochloride tablets 5, 10, or 20 mg/day. The 684 ADHD patients (ages 6 to 17 years) were evaluated in two controlled clinical studies, two clinical pharmacology studies, and two uncontrolled long-term safety studies. Safety data on all patients are included in the discussion that follows. Adverse reactions were assessed by collecting adverse events, and results of physical examinations, vital sign and body weight measurements, and laboratory analyses.
Adverse events during exposure were primarily obtained by general inquiry and recorded by clinical investigators using terminology of their own choosing. Consequently, it is not possible to provide a meaningful estimate of the proportion of individuals experiencing adverse events without first grouping similar types of events into a smaller number of standardized event categories. In the tables and tabulations that follow, standard COSTART dictionary terminology has been used to classify reported adverse events.
The stated frequencies of adverse events represent the proportion of individuals who experienced, at least once, a treatment-emergent adverse event of the type listed. An event was considered treatment emergent if it occurred for the first time or worsened while receiving therapy following baseline evaluation.
Adverse Findings in Clinical Trials with Dexmethylphenidate Hydrochloride Tablets
Adverse Events Associated with Discontinuation of Treatment
No dexmethylphenidate hydrochloride tablets-treated patients discontinued due to adverse events in two placebo-controlled trials. Overall, 50 of 684 children treated with dexmethylphenidate hydrochloride tablets (7.3%) experienced an adverse event that resulted in discontinuation. The most common reasons for discontinuation were twitching (described as motor or vocal tics), anorexia, insomnia, and tachycardia (approximately 1% each).
Adverse Events Occurring at an Incidence of 5% or More Among Dexmethylphenidate Hydrochloride Tablets-Treated Patients
Table 1 enumerates treatment-emergent adverse events for two, placebo-controlled, parallel group trials in children with ADHD at dexmethylphenidate hydrochloride tablets doses of 5, 10, and 20 mg/day. The table includes only those events that occurred in 5% or more of patients treated with dexmethylphenidate hydrochloride tablets where the incidence in patients treated with dexmethylphenidate hydrochloride tablets was at least twice the incidence in placebo-treated patients. The prescriber should be aware that these figures cannot be used to predict the incidence of adverse events in the course of usual medical practice where patient characteristics and other factors differ from those which prevailed in the clinical trials. Similarly, the cited frequencies cannot be compared with figures obtained from other clinical investigations involving different treatments, uses, and investigators. The cited figures, however, do provide the prescribing physician with some basis for estimating the relative contribution of drug and non-drug factors to the adverse event incidence rate in the population studied.
Table 1 Treatment-Emergent Adverse Events1 Occurring During Double-Blind Treatment in Clinical Trials of Dexmethylphenidate Hydrochloride Tablets
|
|
Preferred Term
|
Dexmethylphenidate hydrochloride tablets
|
Placebo
|
|
Body as a Whole
|
|
|
|
|
Digestive System
|
|
|
|
|
|
Anorexia
|
6%
|
1%
|
|
|
Nausea
|
9%
|
1%
|
1Events, regardless of causality, for which the incidence for patients treated with dexmethylphenidate hydrochloride tablets was at least 5% and twice the incidence among placebo-treated patients. Incidence has been rounded to the nearest whole number.
Adverse Events from Postmarketing Experience
The following additional adverse reactions have been identified during postapproval use of dexmethylphenidate hydrochloride extended-release capsules. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency:
Musculoskeletal: rhabdomyolysis
Immune System Disorders: hypersensitivity reactions, including angioedema and anaphylaxis
Adverse Events with Other Methylphenidate HCl Products
Nervousness and insomnia are the most common adverse reactions reported with other methylphenidate products. In children, loss of appetite, abdominal pain, weight loss during prolonged therapy, insomnia, and tachycardia may occur more frequently; however, any of the other adverse reactions listed below may also occur.
Other reactions include:
Cardiac: angina, arrhythmia, palpitations, pulse increased or decreased, tachycardia
Gastrointestinal: nausea
Immune: hypersensitivity reactions including skin rash, urticaria, fever, arthralgia, exfoliative dermatitis, erythema multiforme with histopathological findings of necrotizing vasculitis, and thrombocytopenic purpura
Nervous System: dizziness, drowsiness, dyskinesia, headache, rare reports of Tourette’s syndrome, serotonin syndrome in combination with serotonergic drugs, toxic psychosis
Vascular: blood pressure increased or decreased, cerebral arteritis and/or occlusion
Although a definite causal relationship has not been established, the following have been reported in patients taking methylphenidate:
Blood/lymphatic: leukopenia and/or anemia
Hepatobiliary: abnormal liver function, ranging from transaminase elevation to severe hepatic injury
Psychiatric: transient depressed mood, aggressive behavior, libido changes
Skin/subcutaneous: scalp hair loss
Urogenital System: priapism
Very rare reports of neuroleptic malignant syndrome (NMS) have been received, and, in most of these, patients were concurrently receiving therapies associated with NMS. In a single report, a 10-year-old boy who had been taking methylphenidate for approximately 18 months experienced an NMS-like event within 45 minutes of ingesting his first dose of venlafaxine. It is uncertain whether this case represented a drug-drug interaction, a response to either drug alone, or some other cause.
In children, loss of appetite, abdominal pain, weight loss during prolonged therapy, insomnia, and tachycardia may occur more frequently; however, any of the other adverse reactions listed above may also occur.
DRUG ABUSE AND DEPENDENCE
Controlled Substance Class
Dexmethylphenidate hydrochloride tablets, like other methylphenidate products, are classified as a Schedule II controlled substance by Federal regulation.
Abuse, Dependence, and Tolerance
See WARNINGS for boxed warning containing drug abuse and dependence information.
OVERDOSAGE
Signs and Symptoms
Signs and symptoms of acute methylphenidate overdosage, resulting principally from overstimulation of the CNS and from excessive sympathomimetic effects, may include the following: vomiting, agitation, tremors, hyperreflexia, muscle twitching, convulsions (may be followed by coma), euphoria, confusion, hallucinations, delirium, sweating, flushing, headache, hyperpyrexia, tachycardia, palpitations, cardiac arrhythmias, hypertension, mydriasis, and dryness of mucous membranes. Rhabdomyolysis has also been reported in overdose.
Recommended Treatment
Treatment consists of appropriate supportive measures. The patient must be protected against self-injury and against external stimuli that would aggravate overstimulation already present. Gastric contents may be evacuated by gastric lavage as indicated. Before performing gastric lavage, control agitation and seizures if present and protect the airway. Other measures to detoxify the gut include administration of activated charcoal and a cathartic. Intensive care must be provided to maintain adequate circulation and respiratory exchange; external cooling procedures may be required for hyperpyrexia.
Efficacy of peritoneal dialysis for dexmethylphenidate hydrochloride tablets overdosage has not been established.
Poison Control Center
As with the management of all overdosage, the possibility of multiple drug ingestion should be considered. The physician may wish to consider contacting a poison control center for up-to-date information on the management of overdosage with methylphenidate.
DOSAGE AND ADMINISTRATION
Dexmethylphenidate hydrochloride tablets are administered twice daily, at least 4 hours apart. Dexmethylphenidate hydrochloride tablets may be administered with or without food.
Dosage should be individualized according to the needs and responses of the patient.
Patients New to Methylphenidate
The recommended starting dose of dexmethylphenidate hydrochloride tablets for patients who are not currently taking racemic methylphenidate, or for patients who are on stimulants other than methylphenidate, is 5 mg/day (2.5 mg twice daily).
Dosage may be adjusted in 2.5 to 5 mg increments to a maximum of 20 mg/day (10 mg twice daily). In general, dosage adjustments may proceed at approximately weekly intervals.
Patients Currently Using Methylphenidate
For patients currently using methylphenidate, the recommended starting dose of dexmethylphenidate hydrochloride tablets is half the dose of racemic methylphenidate. The maximum recommended dose is 20 mg/day (10 mg twice daily).
Maintenance/Extended Treatment
There is no body of evidence available from controlled trials to indicate how long the patient with ADHD should be treated with dexmethylphenidate hydrochloride tablets. It is generally agreed, however, that pharmacological treatment of ADHD may be needed for extended periods. Nevertheless, the physician who elects to use dexmethylphenidate hydrochloride tablets for extended periods in patients with ADHD should periodically re-evaluate the long-term usefulness of the drug for the individual patient with periods off medication to assess the patient’s functioning without pharmacotherapy. Improvement may be sustained when the drug is either temporarily or permanently discontinued.
Dose Reduction and Discontinuation
If paradoxical aggravation of symptoms or other adverse events occur, the dosage should be reduced, or, if necessary, the drug should be discontinued.
If improvement is not observed after appropriate dosage adjustment over a 1-month period, the drug should be discontinued.
HOW SUPPLIED
Dexmethylphenidate hydrochloride tablets are available as follows:
5 mg: Light yellow colored, round, biconvex, beveled edge, uncoated, tablets debossed ‘378’ on one side and ‘S’ above ‘5’ on the other side.
NDC 57664-378-83 Bottles of 30 CRC
NDC 57664-378-88 Bottles of 100 CRC
NDC 57664-378-08 Bottles of 100
NDC 57664-378-13 Bottles of 500
NDC 57664-378-18 Bottles of 1000
NDC 69189-0634-1 single dose pack with 1 tablet as repackaged by Avera McKennan Hospital
Dispense in a tight, light-resistant container as defined in the USP with a child-resistant closure.
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Protect from light and moisture.
PHARMACIST: DISPENSE WITH MEDICATION GUIDE PROVIDED SEPARATELY.
REFERENCE
American Psychiatric Association. Diagnosis and Statistical Manual of Mental Disorders. 4th ed. Washington DC: American Psychiatric Association 1994.
MEDICATION GUIDE
Dexmethylphenidate Hydrochloride Tablets CII
’deks meth" l-fen 'i-dat -
'hī-(
׀)drō
'klō(ə)r-
׀īd
Read the Medication Guide that comes with dexmethylphenidate hydrochloride tablets before you or your child starts taking it and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking to your doctor about your or your child’s treatment with dexmethylphenidate hydrochloride tablets.
|
What is the most important information I should know about dexmethylphenidate hydrochloride tablets? The following have been reported with use of dexmethylphenidate hydrochloride and other stimulant medicines. 1. Heart-related problems: • sudden death in patients who have heart problems or heart defects • stroke and heart attack in adults • increased blood pressure and heart rate Tell your doctor if you or your child have any heart problems, heart defects, high blood pressure, or a family history of these problems. Your doctor should check you or your child carefully for heart problems before starting dexmethylphenidate hydrochloride tablets. Your doctor should check your or your child’s blood pressure and heart rate regularly during treatment with dexmethylphenidate hydrochloride tablets. Call your doctor right away if you or your child has any signs of heart problems such as chest pain, shortness of breath, or fainting while taking dexmethylphenidate hydrochloride tablets. 2. Mental (Psychiatric) problems: All Patients • new or worse behavior and thought problems • new or worse bipolar illness • new or worse aggressive behavior or hostility Children and Teenagers • new psychotic symptoms (such as hearing voices, believing things that are not true, are suspicious) or new manic symptoms Tell your doctor about any mental problems you or your child have, or about a family history of suicide, bipolar illness, or depression. Call your doctor right away if you or your child have any new or worsening mental symptoms or problems while taking dexmethylphenidate hydrochloride tablets, especially seeing or hearing things that are not real, believing things that are not real, or are suspicious. 3. Circulation problems in fingers and toes [Peripheral vasculopathy, including Raynaud's phenomenon]: fingers or toes may feel numb, cool, painful, and/or may change color from pale, to blue, to red • Tell your doctor if you have or your child has numbness, pain, skin color change, or sensitivity to temperature in the fingers or toes. • Call your doctor right away if you have or your child has any signs of unexplained wounds appearing on fingers or toes while taking dexmethylphenidate hydrochloride tablets |
What are dexmethylphenidate hydrochloride tablets?
Dexmethylphenidate hydrochloride tablets are a central nervous system stimulant prescription medicine. They are used for the treatment of attention deficit and hyperactivity disorder (ADHD).
Dexmethylphenidate hydrochloride tablets may help increase attention and decrease impulsiveness and hyperactivity in patients with ADHD. Dexmethylphenidate hydrochloride tablets should be used as a part of a total treatment program for ADHD that may include counseling or other therapies.
Dexmethylphenidate hydrochloride tablets should be used as a part of a total treatment program for ADHD that may include counseling or other therapies.
|
Dexmethylphenidate hydrochloride tablets are a federally controlled substance (CII) because it can be abused or lead to dependence. Keep dexmethylphenidate hydrochloride tablets in a safe place to prevent misuse and abuse. Selling or giving away dexmethylphenidate hydrochloride tablets may harm others, and is against the law. Tell your doctor if you or your child have (or have a family history of) ever abused or been dependent on alcohol, prescription medicines or street drugs. |
Who should not take dexmethylphenidate hydrochloride tablets?
Dexmethylphenidate hydrochloride tablets should not be taken if you or your child:
• are very anxious, tense, or agitated
• have an eye problem called glaucoma
• have tics or Tourette’s syndrome, or a family history of Tourette’s syndrome. Tics are hard to control repeated movements or sounds.
• are taking or have taken within the past 14 days an anti-depression medicine called a monoamine oxidase
inhibitor or MAOI.
• are allergic to anything in dexmethylphenidate hydrochloride tablets. See the end of this Medication
Guide for a complete list of ingredients.
Dexmethylphenidate hydrochloride tablets should not be used in children less than 6 years old because it has not been studied in this age group.
Dexmethylphenidate hydrochloride tablets may not be right for you or your child. Before starting
dexmethylphenidate hydrochloride tablets tell your or your child’s doctor about all health conditions (or a family history of) including:
• heart problems, heart defects, high blood pressure
• mental problems including psychosis, mania, bipolar illness, or depression
• tics or Tourette’s syndrome
• seizures or have had an abnormal brain wave test (EEG)
• circulation problems in fingers or toes
Tell your doctor if you or your child is pregnant, planning to become pregnant, or breast-feeding.
Can dexmethylphenidate hydrochloride tablets be taken with other medicines?
Tell your doctor about all of the medicines that you or your child takes including prescription and nonprescription medicines, vitamins, and herbal supplements. Dexmethylphenidate hydrochloride tablets and some medicines may interact with each other and cause serious side effects. Sometimes the doses of other medicines will need to be adjusted while taking dexmethylphenidate hydrochloride tablets.
Your doctor will decide whether dexmethylphenidate hydrochloride tablets can be taken with other medicines.
Especially tell your doctor if you or your child takes:
• anti-depression medicines including MAOIs
• seizure medicines
• blood thinner medicines
• blood pressure medicines
• cold or allergy medicines that contain decongestants
Know the medicines that you or your child takes. Keep a list of your medicines with you to show your doctor and pharmacist.
Do not start any new medicine while taking dexmethylphenidate hydrochloride tablets without talking to your doctor first.
How should dexmethylphenidate hydrochloride tablets be taken?
• Take dexmethylphenidate hydrochloride tablets exactly as prescribed. Your doctor may adjust the dose until it is right for you or your child.
• Take dexmethylphenidate hydrochloride tablets twice a day, at least 4 hours apart.
• Dexmethylphenidate hydrochloride tablets can be taken with or without food.
• From time to time, your doctor may stop dexmethylphenidate hydrochloride tablets treatment for a while to check ADHD symptoms.
• Your doctor may do regular checks of the blood, heart, and blood pressure while taking dexmethylphenidate hydrochloride tablets. Children should have their height and weight checked often while taking dexmethylphenidate hydrochloride tablets. Dexmethylphenidate hydrochloride tablets treatment may be stopped if a problem is found during these check-ups.
• If you or your child takes too many dexmethylphenidate hydrochloride tablets or overdoses, call your doctor or poison control center right away, or get emergency treatment.
What are possible side effects of dexmethylphenidate hydrochloride tablets?
See “What is the most important information I should know about dexmethylphenidate hydrochloride tablets?” for information on reported heart and mental problems.
Other serious side effects include:
• serious allergic reactions (symptoms can be difficulty breathing, swelling of the face, neck and throat, rashes and hives, fever)
• slowing of growth (height and weight) in children
• seizures, mainly in patients with a history of seizures
• eyesight changes or blurred vision
• painful and prolonged erections (priapism) have occurred with methylphenidate. If you or your child develop priapism, seek medical help right away. Because of the potential for lasting damage, priapism should be evaluated by a doctor immediately.
Common side effects include:
- • stomach ache • decreased appetite
- • nausea • fever
Talk to your doctor if you or your child has side effects that are bothersome or do not go away.
This is not a complete list of possible side effects. Ask your doctor or pharmacist for more information.
How should I store dexmethylphenidate hydrochloride tablets?
• Store dexmethylphenidate hydrochloride tablets in a safe place at room temperature, 20° to 25°C (68° to 77°F).
• Keep dexmethylphenidate hydrochloride tablets and all medicines out of the reach of children.
General information about dexmethylphenidate hydrochloride tablets
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use dexmethylphenidate hydrochloride tablets for a condition for which it was not prescribed. Do not give dexmethylphenidate hydrochloride tablets to other people, even if they have the same condition. It may harm them and it is against the law.
This Medication Guide summarizes the most important information about dexmethylphenidate hydrochloride tablets. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about dexmethylphenidate hydrochloride tablets that was written for healthcare professionals. For more information about dexmethylphenidate hydrochloride tablets, please contact Sun Pharmaceutical Industries, Inc. at 1-800-406-7984.
What are the ingredients in dexmethylphenidate hydrochloride tablets?
Active Ingredient: dexmethylphenidate hydrochloride
Inactive Ingredients: lactose monohydrate, sodium starch glycolate, talc, magnesium stearate, and FD&C Blue No.1 aluminum lake (2.5 mg tablet), D&C Yellow No. 10 aluminum lake (5 mg tablet); the 10 mg tablet contains no dye.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Manufactured and Distributed by:
Sun Pharmaceutical Industries, Inc.
Cranbury, NJ 08512
6487T02
Rev. 01/2017
| DEXMETHYLPHENIDATE HYDROCHLORIDE
dexmethylphenidate hydrochloride tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Avera McKennan Hospital (068647668) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Avera McKennan Hospital | 068647668 | relabel(69189-0634) , repack(69189-0634) | |