AA TOP MEDICATED- menthol and methyl salicylate oil
Albert Max, Inc.
----------
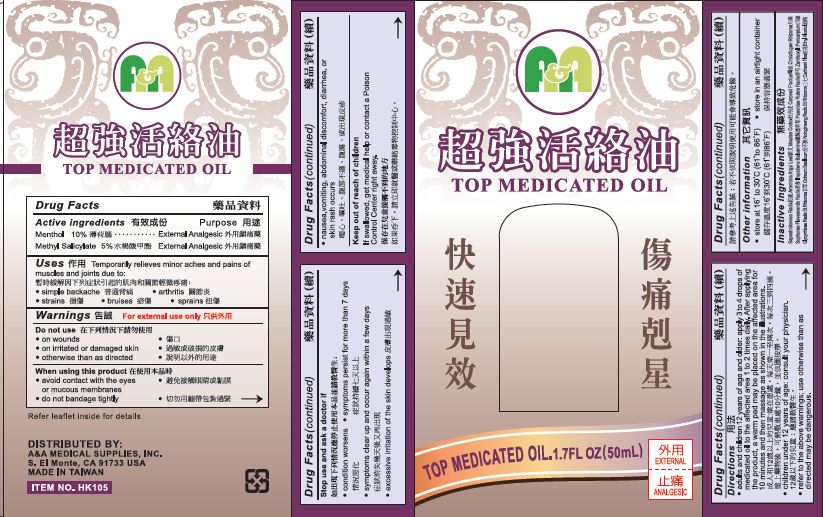
A&A Top Medicated Oil
Uses
Temporarily relieves minor aches and pains of muscles and joints due to:
- simple backache
- arthritis
- strains
- bruises
- sprains
Warnings
For external Use Only
Directions
- adults and children 12 years of age and older: apply 3 to 4 drops of medicated oil to the affected area 1 or 2 times daily. After applying the product, a warm pad may be placed on the affected area for 10 minutes and then massage as shown in the illustrations.
- children under 12 years of age: consult your physician.
- refer to the above warnings; use otherwise than directed may be dangerous.
Inactive Ingredients
Saposhnikoviae Radix, Artemisia Argyi Leaf, Moutan Cortex, Carpesii Fructus, Cimicifugae Rhizoma, Sophorae Flavescentis Radix, Impatiens Balsamina, Paeoniae Rubra Radix, Zanthoxyli Pericarpium, Glycyrrhizae Radix Et Rhizoma, Ocimum Basilicum, Notoginseng Radix Et Rhizoma, Carthami Flos, Ethyl Alcohol
| AA TOP MEDICATED
menthol and methyl salicylate oil |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Albert Max, Inc. (149445798) |
Revised: 10/2023
Document Id: 074c03eb-4f98-1d0c-e063-6394a90a3aaa
Set id: 6193cc31-ab84-3240-e053-2991aa0a6ffb
Version: 6
Effective Time: 20231009
Albert Max, Inc.