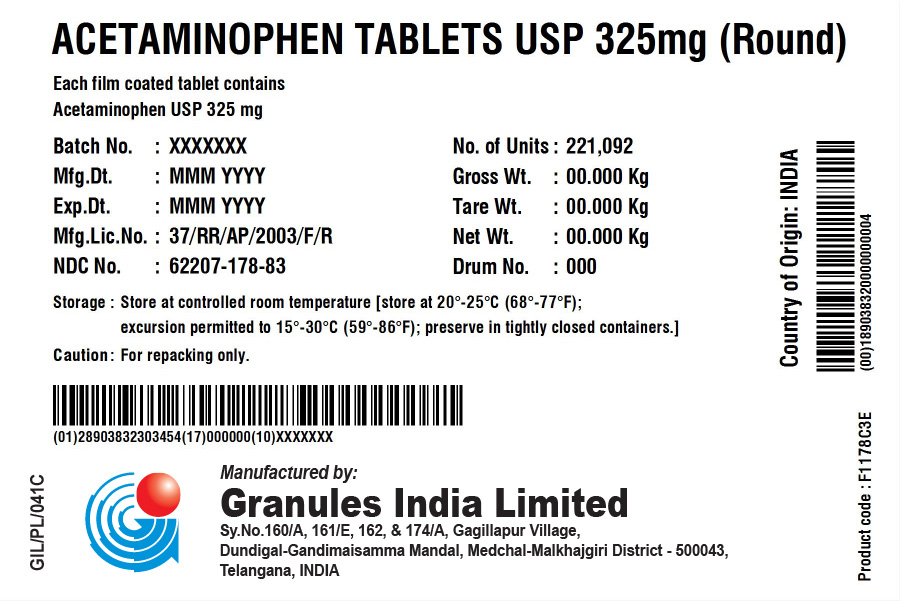
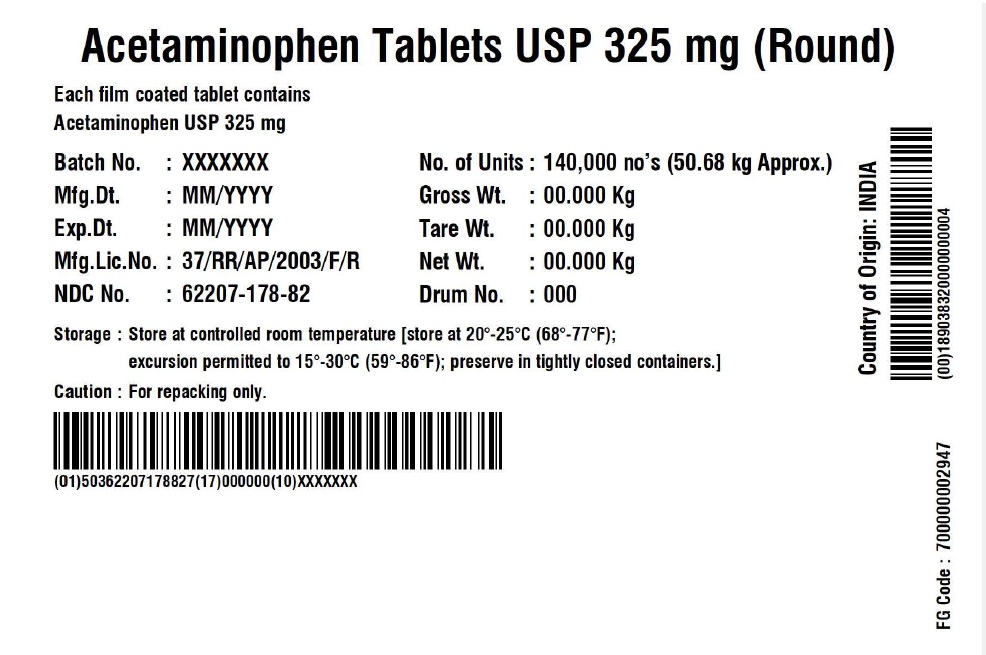
Label: ACETAMINOPHEN 325 MG ROUND- acetaminophen tablet
- NDC Code(s): 62207-178-82, 62207-178-83
- Packager: Granules India Limited
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated January 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Acetaminophen Tablets 325 mg
-
INGREDIENTS AND APPEARANCE
ACETAMINOPHEN 325 MG ROUND
acetaminophen tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62207-178 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) STARCH, CORN (UNII: O8232NY3SJ) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) POVIDONE K30 (UNII: U725QWY32X) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color white (white to Off-White) Score no score Shape ROUND Size 9mm Flavor Imprint Code G323 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62207-178-83 221092 in 1 BOX; Type 0: Not a Combination Product 12/29/2017 2 NDC:62207-178-82 140000 in 1 DRUM; Type 0: Not a Combination Product 04/30/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 12/29/2017 Labeler - Granules India Limited (915000087)