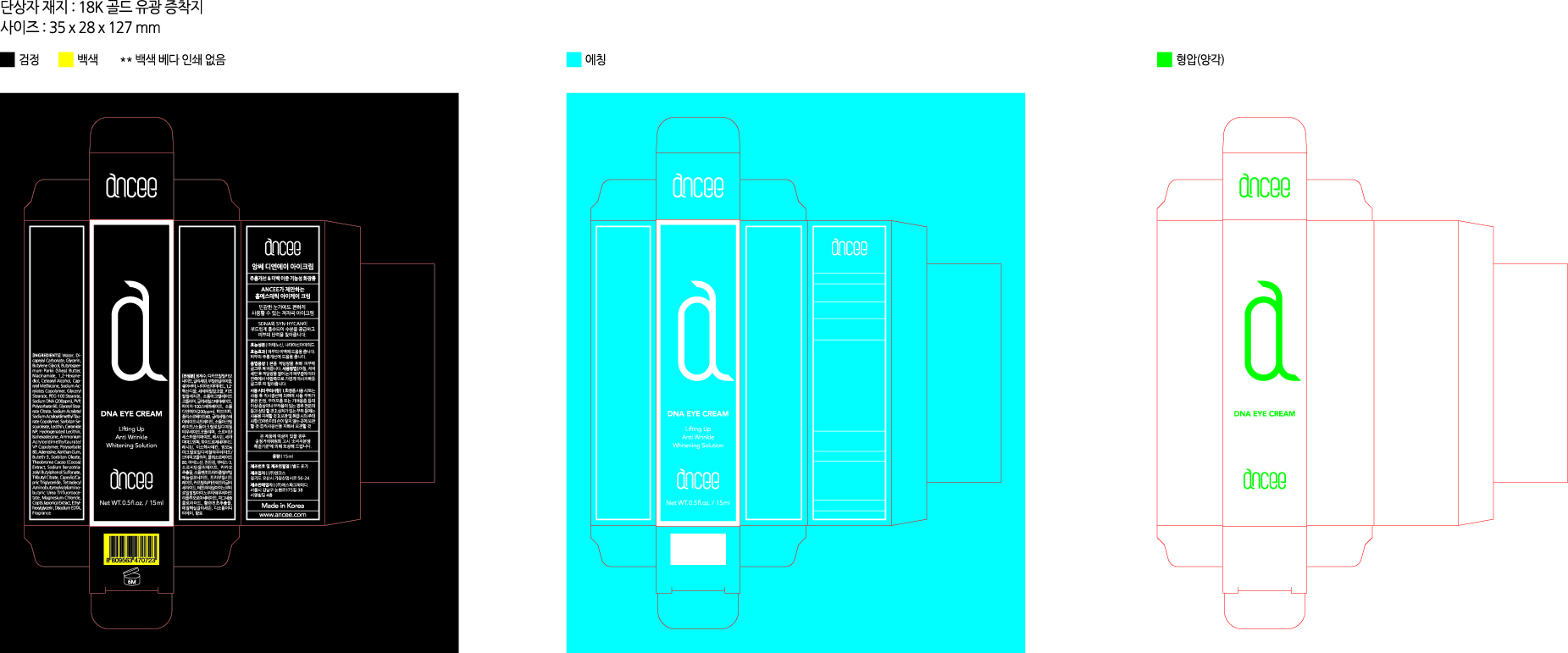
ANCEE DNA EYE- glycerin cream
SPARKJD Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Dicaprylyl Carbonate, Butylene Glycol, Butyrospermum Parkii (Shea) Butter, Niacinamide, Adenosine, etc.
After morning and evening cleansing, tone your face and take the right amount of ANCEE EYE CREAM and gently spread out on eye zone. Tap lightly on the eye zone until the cream is absorbed into skin.
| ANCEE DNA EYE
glycerin cream |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - SPARKJD Inc. (688353975) |
| Registrant - SPARKJD Inc. (688353975) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| SPARKJD Inc. | 688353975 | label(71933-0001) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| ANCORS CO., LTD. | 688494804 | manufacture(71933-0001) | |
Revised: 9/2021
Document Id: cbec26ad-0040-853b-e053-2a95a90ad821
Set id: 60bd4e9a-e080-a533-e053-2991aa0a7983
Version: 4
Effective Time: 20210913
SPARKJD Inc.