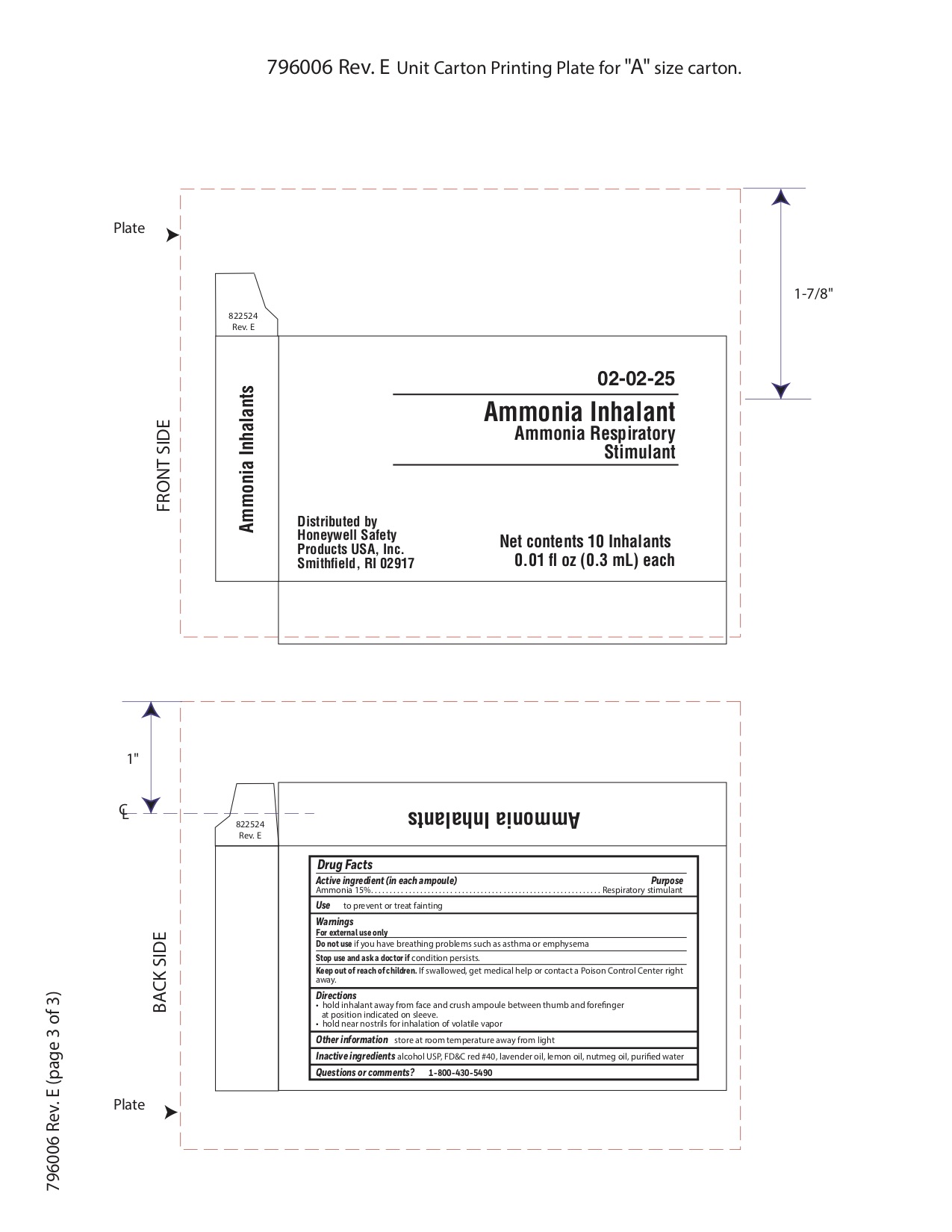
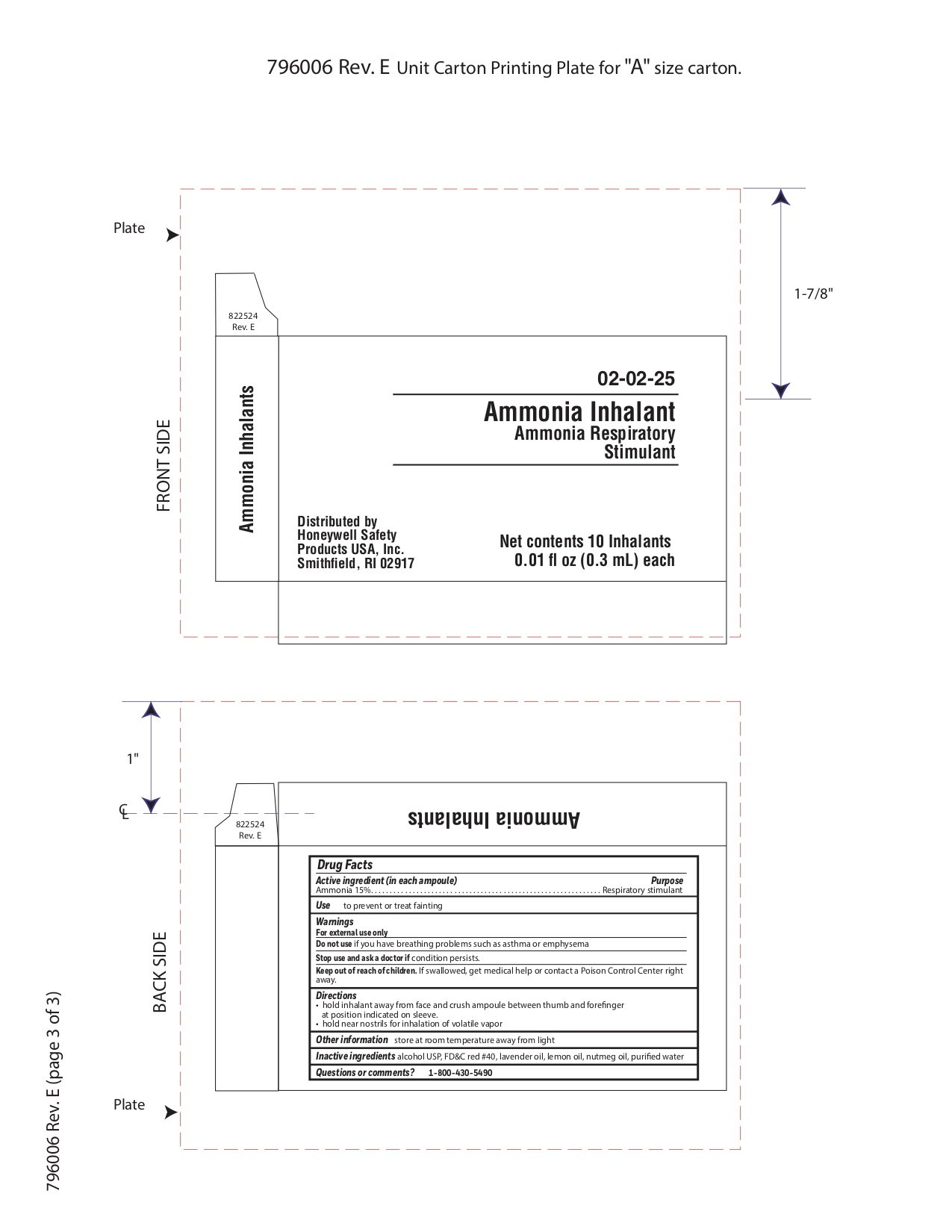
Active ingredient (in each ampoule)
Ammonia 15%
Purpose
Respiratory stimulant
Uses
to prevent or treat fainting
Warnings
For external use only
Do not use
- if you have breathing problems such as asthma or emphysema
Stop use and ask a doctor if
Keep out of reach of children
If swallowed get medical help or contact a Poison Control Center right away.
Directions
- hold inhalant away from face and crush ampoule between thumb and forefinger at position indicated on sleeve.
- hold near nostrils for inhalation of volatile vapor
Other information
store at room temperature away from light
Inactive ingredients
alcohol USP, FD&C red #40, lavender oil, lemon oil fcc, nutmeg oil, purified water
Questions or comments?
1-800-430-5490
Principal Display Panel
Honeywell Ammonia