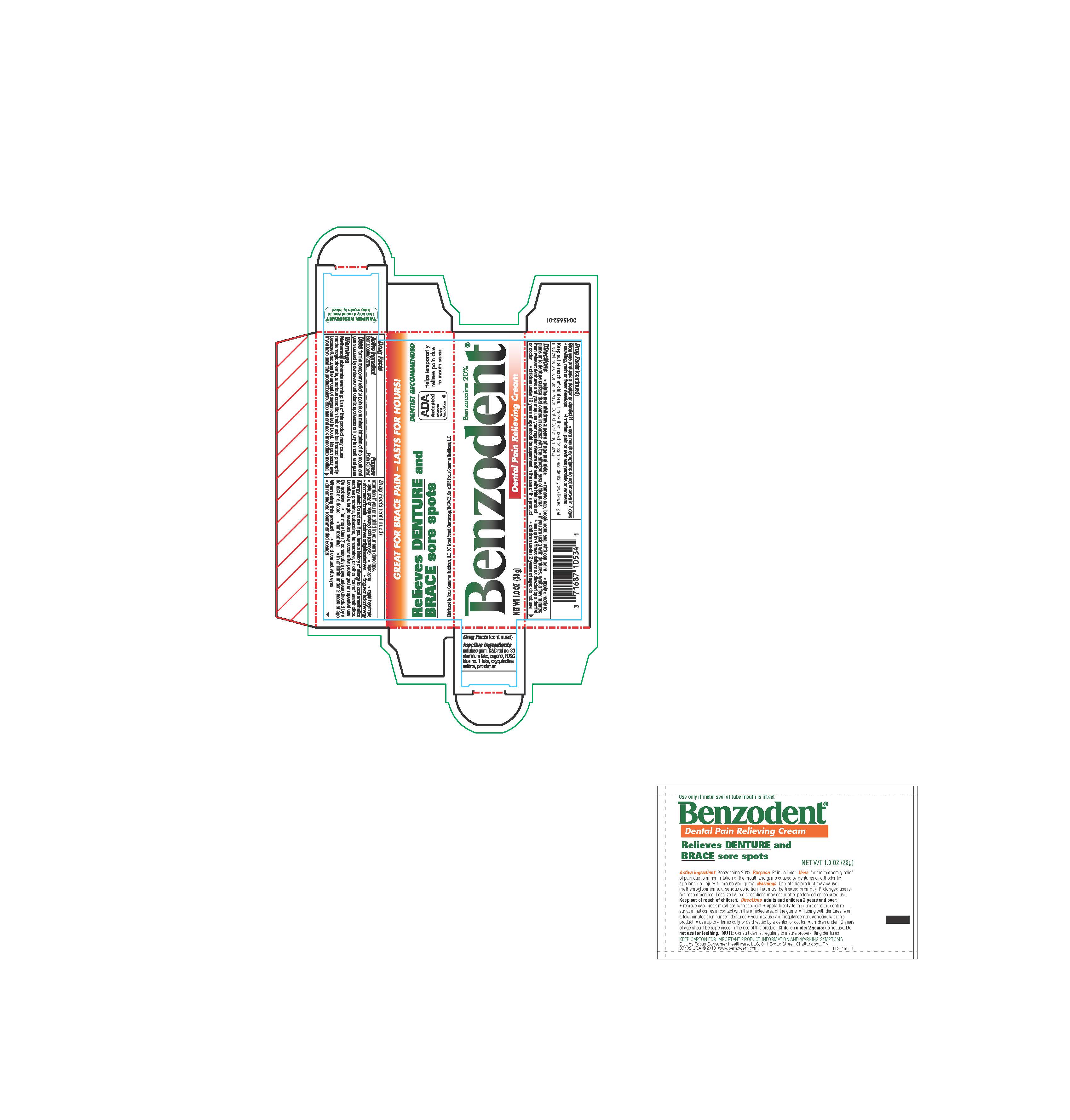
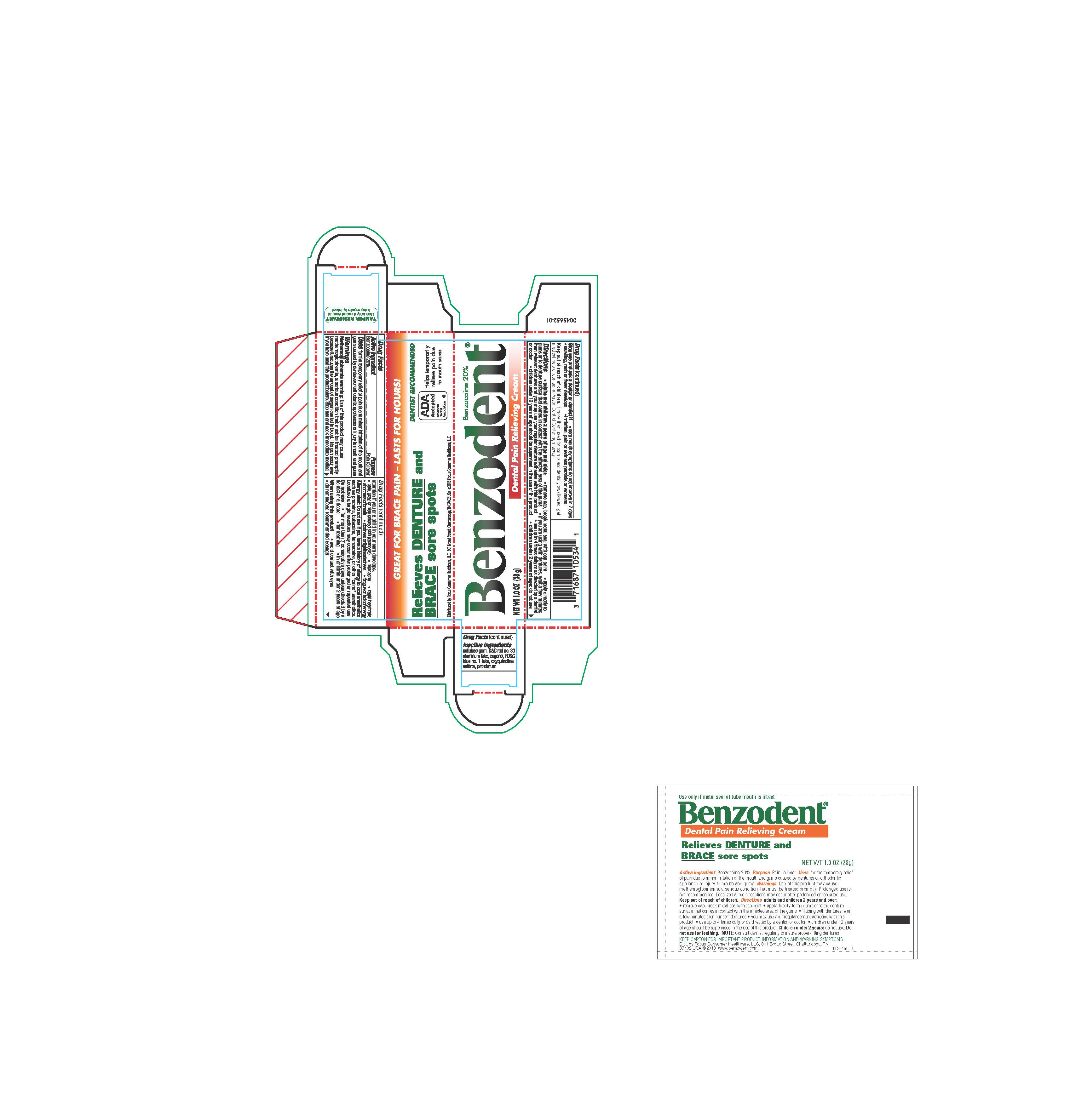
Label: BENZODENT- benzocaine cream
- NDC Code(s): 71687-1053-1, 71687-1053-2
- Packager: Focus Consumer Healthcare, LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 12, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
DOSAGE & ADMINISTRATION
Adults and children12 years and over:
- remove cap, break metal seal with cap point
- apply directly to the gums or to the denture surface that comes in contact with the affected area of the gums
- if you are using the dentures, wait a few minutes then reinsert dentures and you may use your regular denture adhesive with this product
- use up to 4 times daily or as directed by a dentist or doctor
- children under 12 years of age should be supervised in the use of this product
children under 12 years: consult a dentist or doctor
-
WARNINGS
Methemoglobinemia warning: Use of this product may cause methemoglobinemia, a serious condition that must be treated promptly because it reduces the amount of oxygen carried to the blood. This can occur even if you have used this product before. Stop use and seek immedicate medical attention if you or a child in your care develops:
- pale, gray or blue colored skin (cyanosis)
- headache
- rapid heart rate
- shortness of breath
- dizziness or lightheadedness
- fatigue or lack of energy
Alllergy alert: Do not use if you have a history of allergy to local anetsthetics such as procaine, butacaine, benzocaine, or other "caine: anesthetics. Localized allergic reactions may occur after prolonged or repeated use.
Do not use
- for more than 7 consecutive days unless directed by a dentist of a doctor
- for teething
- in children under 2 years of age
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BENZODENT
benzocaine creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71687-1053 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 0.2 g in 1 g Inactive Ingredients Ingredient Name Strength FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C RED NO. 30 (UNII: 2S42T2808B) EUGENOL (UNII: 3T8H1794QW) ALUMINUM OXIDE (UNII: LMI26O6933) OXYQUINOLINE SULFATE (UNII: 61VUG75Y3P) PETROLATUM (UNII: 4T6H12BN9U) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71687-1053-1 1 in 1 CARTON 12/08/2017 1 7 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:71687-1053-2 1 in 1 CARTON 12/08/2017 2 28 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 12/08/2017 Labeler - Focus Consumer Healthcare, LLC (080743737)