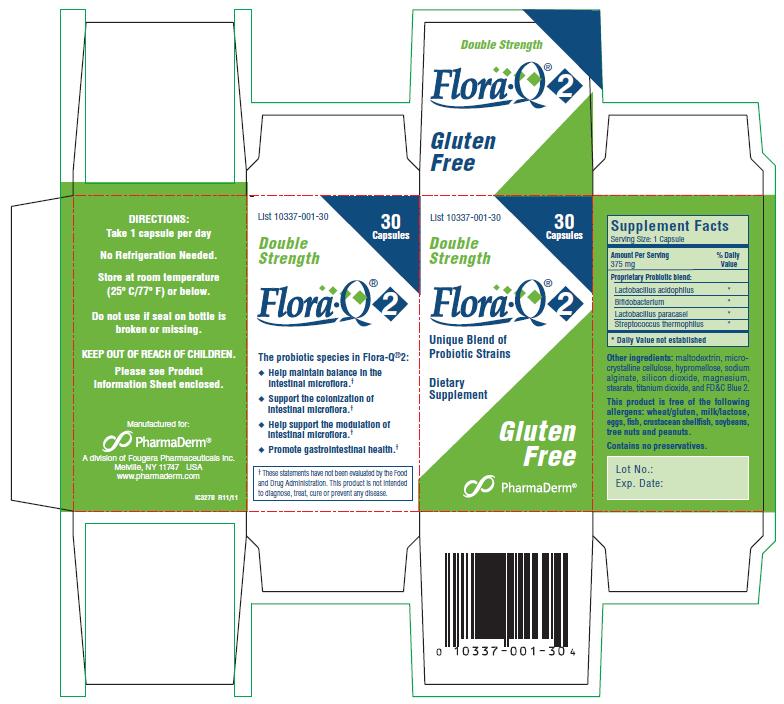
Label: FLORA Q2- lactobacillus acidophilus capsule
- NHRIC Code(s): 10337-001-30
- Packager: PharmaDerm A division of Fougera Pharmaceuticals Inc.
- Category: DIETARY SUPPLEMENT
Drug Label Information
Updated May 7, 2012
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
STATEMENT OF IDENTITY
PRODUCT DESCRIPTION: Off-white capsules, each containing 300 mg* of a standardized, light-beige colored powder consisting of an aggregate of a minimum of 16 billion CFU (16 x 109 Colony Forming Units) freeze-dried cultures at time of manufacture of species listed below.
Guaranteed potency of no less than 8 billion CFU freeze-dried cultures at expiration date.
* Based on an estimated tapped density of 0.7 g/mL.
Also contains: maltodextrin, hypromellose, microcrystalline cellulose, sodium alginate, silicon dioxide, magnesium stearate, titanium dioxide, and FD&C Blue 2.
Each capsule, containing a proprietary probiotic blend and other ingredients, is equivalent to 375 mg per serving.
This product is free of the following allergens: wheat/gluten, milk/lactose, eggs, fish, crustacean shellfish, soybeans, tree nuts, and peanuts.
Contains no preservatives.
GENERIC NAME:
Lactobacillus acidophilus
Bifidobacterium
Lactobacillus paracasei
Streptococcus thermophilus
-
HEALTH CLAIM
COMMON USES: This probiotic is used to help maintain the normal bacterial balance of the gastrointestinal tract.†
THE PROBIOTIC SPECIES IN FLORA•Q®2:
- Help maintain balance in the intestinal microflora.†
- Support the colonization of intestinal microflora.†
- Help support the modulation of intestinal microflora.†
- Promote gastrointestinal health.†
†These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease. -
PRECAUTIONS
HOW TO USE: Use this product as directed on the package, unless instructed differently by your doctor.
CAUTIONS: Before you begin taking any dietary supplement, check with your doctor or pharmacist.
KEEP OUT OF THE REACH OF CHILDREN.
POSSIBLE SIDE EFFECTS: Potential side effects that may dissipate include increased stomach gas. If this occurs and is bothersome, check with your doctor. Should you experience symptoms of an allergic reaction, including rash, itching, swelling, dizziness or trouble breathing, contact your doctor, nurse or pharmacist immediately.
- DOSAGE & ADMINISTRATION
-
SAFE HANDLING WARNING
HOW SUPPLIED: Off-white capsules imprinted Flora•Q®2 are supplied in bottles of 30 (List 10337-001-30).
STORAGE: Potency guaranteed through expiration date on package when stored at room temperature (25º C/77º F) or below. DO NOT STORE IN THE BATHROOM. Keep container closed. No refrigeration needed.
Do not use if seal on bottle is broken or missing.
Manufactured for:
PharmaDerm®
A divisdion of
Fougera
PHARMEUTICALS INC.
Melville, New York 11747
www.pharmaderm.comIL306B R11/11
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FLORA Q2
lactobacillus acidophilus capsuleProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:10337-001 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength lactobacillus acidophilus (UNII: 1PRR1V42V5) (lactobacillus acidophilus - UNII:1PRR1V42V5) lactobacillus acidophilus 16000000000 [CFU] Inactive Ingredients Ingredient Name Strength maltodextrin (UNII: 7CVR7L4A2D) hypromelloses (UNII: 3NXW29V3WO) cellulose, microcrystalline (UNII: OP1R32D61U) sodium alginate (UNII: C269C4G2ZQ) silicon dioxide (UNII: ETJ7Z6XBU4) magnesium stearate (UNII: 70097M6I30) titanium dioxide (UNII: 15FIX9V2JP) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:10337-001-30 1 in 1 CARTON 1 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Dietary Supplement 08/01/2007 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color shape size (solid drugs) 16 mm scoring 2 imprint Labeler - PharmaDerm A division of Fougera Pharmaceuticals Inc. (043838424)