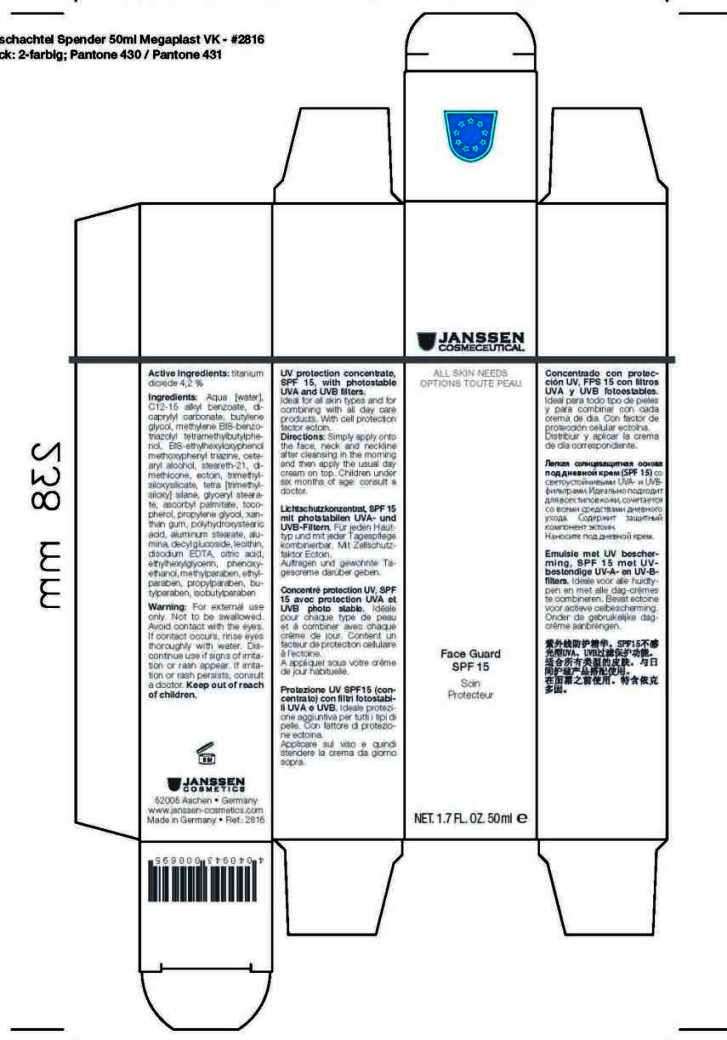
Label: FACE GUARD 15- titanium dioxide cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 24653-816-01, 24653-816-02 - Packager: Janssen Cosmetics GmbH
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 31, 2012
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
-
Inactive Ingredients
Aqua [water], C12-15 alkyl benzoate, dicaprylyl carbonate, butylenes glycol, methylene BIS-benzotriazolyl tetramethylbutylphenol, BIS-ethylhexyloxyphenol methoxyphenyl triazine, cetearyl alcohol, steareth-21, dimethicone, ectoin, trimethylsiloxysilicate, tetra [trimethylsiloxy] silane, glyceryl stearate, ascorbyl palmitate, tocopherol, propylene glycol, xanthan gum, polyhydroxystearic acid, aluminum stearate, alumina, decyl glucoside, lecithin, disodium EDTA, citric acid, ethylhexylglycerin, phenoxyethanol, methylparaben, ethylparaben, propylparaben, butylparaben, isobutylparaben
- Ask Doctor Section
- Warnings
- Directions
- Front Panel of Box
-
INGREDIENTS AND APPEARANCE
FACE GUARD 15
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:24653-816 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 4.2 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) BISOCTRIZOLE (UNII: 8NT850T0YS) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) BEMOTRIZINOL (UNII: PWZ1720CBH) STEARETH-21 (UNII: 53J3F32P58) DIMETHICONE (UNII: 92RU3N3Y1O) ECTOINE (UNII: 7GXZ3858RY) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) GLYCERETH-17 STEARATE (UNII: 02R46S8KUH) ALUMINUM STEARATE (UNII: U6XF9NP8HM) ALUMINUM OXIDE (UNII: LMI26O6933) XANTHAN GUM (UNII: TTV12P4NEE) ASCORBYL PALMITATE (UNII: QN83US2B0N) TOCOPHEROL (UNII: R0ZB2556P8) DECYL GLUCOSIDE (UNII: Z17H97EA6Y) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) EDETIC ACID (UNII: 9G34HU7RV0) PROPYLENE (UNII: AUG1H506LY) CITRIC ACID ACETATE (UNII: DSO12WL7AU) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) BUTYLPARABEN (UNII: 3QPI1U3FV8) ETHYLPARABEN (UNII: 14255EXE39) ISOBUTYLPARABEN (UNII: 0QQJ25X58G) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:24653-816-02 1 in 1 BOX 1 NDC:24653-816-01 50 mL in 1 BOTTLE, PUMP Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 10/02/2012 Labeler - Janssen Cosmetics GmbH (499187946) Registrant - Janssen Cosmetics GmbH (499187946) Establishment Name Address ID/FEI Business Operations Janssen Cosmetics Gmbh 499187946 manufacture(24653-816)