DIPHENHYDRAMINE HCL- diphenhydramine hcl capsule, liquid filled
AiPing Pharmaceutical, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Nighttime Sleep Aid
Diphenhydramine HCl Softgels, USP 50 mg
Warnings
Do not use
- for children under 12 years of age
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
Directions
- adults and children 12 years of age and over: 1 softgel (50 mg) at bedtime if needed, or as directed by a doctor
Inactive ingredients
FD&C blue #1, gelatin, glycerin, polyethylene glycol, propylene glycol, purified water, sorbitol sorbitan solution and white edible ink
PRINCIPAL DISPLAY PANEL
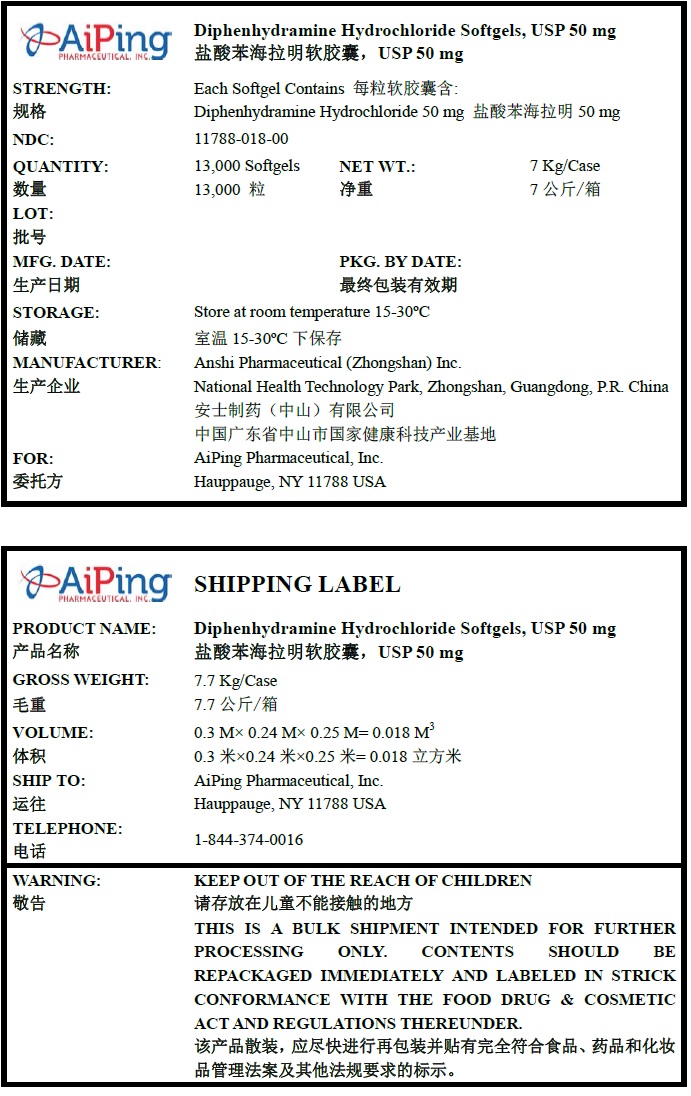
Diphenhydramine Hydrochloride Softgels, USP 50 mg
Quantity : 13000 Softgels
NDC. No : 11788-018-00
WARNING:
KEEP OUT OF THE REACH OF CHILDREN. THIS IS A BULK SHIPMENT INTENDED FOR FURTHER PROCESSING ONLY. CONTENTS SHOULD BE REPACKAGED IMMEDIATELY AND LABELED IN STRICK CONFORMANCE WITH THE FOOD DRUG & COSMETIC ACT AND REGULATIONS THEREUNDER.
| DIPHENHYDRAMINE HCL
diphenhydramine hcl capsule, liquid filled |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - AiPing Pharmaceutical, Inc. (079674526) |
| Registrant - AiPing Pharmaceutical, Inc. (079674526) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Anshi Pharmaceutical (Zhongshan) Inc. | 528101821 | manufacture(11788-018) | |