Label: SANDIMMUNE- cyclosporine capsule, liquid filled
SANDIMMUNE- cyclosporine injection
SANDIMMUNE- cyclosporine solution
-
NDC Code(s):
0078-0109-01,
0078-0109-61,
0078-0110-22,
0078-0240-15, view more0078-0240-61, 0078-0241-15, 0078-0241-61
- Packager: Novartis Pharmaceuticals Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated January 9, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
WARNING
Only physicians experienced in immunosuppressive therapy and management of organ transplant patients should prescribe Sandimmune (cyclosporine). Patients receiving the drug should be managed in facilities equipped and staffed with adequate laboratory and supportive medical resources. The physician responsible for maintenance therapy should have complete information requisite for the follow-up of the patient.
Sandimmune (cyclosporine) should be administered with adrenal corticosteroids but not with other immunosuppressive agents. Increased susceptibility to infection and the possible development of lymphoma may result from immunosuppression.
Sandimmune Soft Gelatin Capsules (cyclosporine capsules, USP) and Sandimmune Oral Solution (cyclosporine oral solution, USP) have decreased bioavailability in comparison to Neoral Soft Gelatin Capsules (cyclosporine capsules, USP) MODIFIED and Neoral Oral Solution (cyclosporine oral solution, USP) MODIFIED.
Sandimmune and Neoral are not bioequivalent and cannot be used interchangeably without physician supervision.
The absorption of cyclosporine during chronic administration of Sandimmune Soft Gelatin Capsules and Oral Solution was found to be erratic. It is recommended that patients taking the soft gelatin capsules or oral solution over a period of time be monitored at repeated intervals for cyclosporine blood concentrations and subsequent dose adjustments be made in order to avoid toxicity due to high concentrations and possible organ rejection due to low absorption of cyclosporine. This is of special importance in liver transplants. Numerous assays are being developed to measure blood concentrations of cyclosporine. Comparison of concentrations in published literature to patient concentrations using current assays must be done with detailed knowledge of the assay methods employed (see DOSAGE AND ADMINISTRATION, Blood Concentration Monitoring).
-
DESCRIPTION
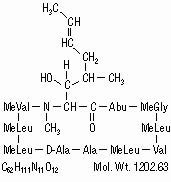
Cyclosporine, the active principle in Sandimmune (cyclosporine) is a cyclic polypeptide immunosuppressant agent consisting of 11 amino acids. It is produced as a metabolite by the fungus species Beauveria nivea.
Chemically, cyclosporine is designated as [R-[R*,R*-(E)]]-cyclic(L-alanyl-D-alanyl-N-methyl-L-leucyl-N-methyl-L-leucyl-N-methyl-L-valyl-3-hydroxy-N,4-dimethyl-L-2-amino-6-octenoyl-L-α-amino-butyryl-N-methylglycyl-N-methyl-L-leucyl-L-valyl-N-methyl-L-leucyl).
Sandimmune® Soft Gelatin Capsules (cyclosporine capsules, USP) are available in 25 mg and 100 mg strengths.
Each 25 mg capsule contains:
cyclosporine, USP…………………………………………………………………………………………25 mg
alcohol, USP dehydrated………………………………………………………………max 12.7% by volume
Each 100 mg capsule contains:
cyclosporine, USP……………………………………………………………………………………….100 mg
alcohol, USP dehydrated………………………………………………………………max 12.7% by volume
Inactive ingredients: corn oil, gelatin, iron oxide red, linoleoyl macrogolglycerides, sorbitol, and titanium dioxide. May also contain glycerol. 100 mg capsules may contain iron oxide yellow.
Sandimmune® Oral Solution (cyclosporine oral solution, USP) is available in 50 mL bottles.
Each mL contains:
cyclosporine, USP……………………………………………………………………………………….100 mg
alcohol, Ph. Helv. ……………………………………………………………………………12.5% by volume
dissolved in an olive oil, Ph. Helv./Labrafil M 1944 CS (polyoxyethylated oleic glycerides) vehicle which must be further diluted with milk, chocolate milk, or orange juice before oral administration.
Sandimmune® Injection (cyclosporine injection, USP) is available in a 5 mL sterile ampul for intravenous (IV) administration.
Each mL contains:
cyclosporine, USP…………………………………………………………………………………………50 mg
*Cremophor® EL (polyoxyethylated castor oil)………………………………………………………..650 mg
alcohol, Ph. Helv. ……………………………………………………………………………32.9% by volume
nitrogen………………………………………………………………………………………………………….qs
which must be diluted further with 0.9% Sodium Chloride Injection or 5% Dextrose Injection before use.
The chemical structure of cyclosporine (also known as cyclosporin A) is
-
CLINICAL PHARMACOLOGY
Cyclosporine is a potent immunosuppressive agent, which in animals prolongs survival of allogeneic transplants involving skin, heart, kidney, pancreas, bone marrow, small intestine, and lung. Cyclosporine has been demonstrated to suppress some humoral immunity and to a greater extent, cell-mediated reactions, such as allograft rejection, delayed hypersensitivity, experimental allergic encephalomyelitis, Freund’s adjuvant arthritis, and graft vs. host disease in many animal species for a variety of organs.
Successful kidney, liver, and heart allogeneic transplants have been performed in man using cyclosporine.
The exact mechanism of action of cyclosporine is not known. Experimental evidence suggests that the effectiveness of cyclosporine is due to specific and reversible inhibition of immunocompetent lymphocytes in the G0- or G1-phase of the cell cycle. T-lymphocytes are preferentially inhibited. The T-helper cell is the main target, although the T-suppressor cell may also be suppressed. Cyclosporine also inhibits lymphokine production and release, including interleukin-2 or T-cell growth factor (TCGF).
No functional effects on phagocytic (changes in enzyme secretions not altered, chemotactic migration of granulocytes, macrophage migration, carbon clearance in vivo) or tumor cells (growth rate, metastasis) can be detected in animals. Cyclosporine does not cause bone marrow suppression in animal models or man.
The absorption of cyclosporine from the gastrointestinal tract is incomplete and variable. Peak concentrations (Cmax) in blood and plasma are achieved at about 3.5 hours. Cmax and area under the plasma or blood concentration/time curve (AUC) increase with the administered dose; for blood, the relationship is curvilinear (parabolic) between 0 and 1400 mg. As determined by a specific assay, Cmax is approximately 1.0 ng/mL/mg of dose for plasma and 2.7 to 1.4 ng/mL/mg of dose for blood (for low to high doses). Compared to an intravenous infusion, the absolute bioavailability of the oral solution is approximately 30% based upon the results in 2 patients. The bioavailability of Sandimmune Soft Gelatin Capsules (cyclosporine capsules, USP) is equivalent to Sandimmune Oral Solution, (cyclosporine oral solution, USP).
Cyclosporine is distributed largely outside the blood volume. In blood, the distribution is concentration dependent. Approximately 33% to 47% is in plasma, 4% to 9% in lymphocytes, 5% to 12% in granulocytes, and 41% to 58% in erythrocytes. At high concentrations, the uptake by leukocytes and erythrocytes becomes saturated. In plasma, approximately 90% is bound to proteins, primarily lipoproteins.
The disposition of cyclosporine from blood is biphasic with a terminal half-life of approximately 19 hours (range, 10 to 27 hours). Elimination is primarily biliary with only 6% of the dose excreted in the urine.
Cyclosporine is extensively metabolized but there is no major metabolic pathway. Only 0.1% of the dose is excreted in the urine as unchanged drug. Of 15 metabolites characterized in human urine, 9 have been assigned structures. The major pathways consist of hydroxylation of the Cγ-carbon of 2 of the leucine residues, Cη-carbon hydroxylation, and cyclic ether formation (with oxidation of the double bond) in the side chain of the amino acid 3-hydroxyl-N,4-dimethyl-L-2-amino-6-octenoic acid and N-demethylation of N-methyl leucine residues. Hydrolysis of the cyclic peptide chain or conjugation of the aforementioned metabolites do not appear to be important biotransformation pathways.
Specific Populations
Renal Impairment
In a study performed in 4 subjects with end-stage renal disease (creatinine clearance < 5mL/min), an intravenous infusion of 3.5 mg/kg of cyclosporine over 4 hours administered at the end of a hemodialysis session resulted in a mean volume of distribution (Vdss) of 3.49 L/kg and systemic clearance (CL) of 0.369 L/hr/kg. This systemic CL (0.369 L/hr/kg) was approximately two thirds of the mean systemic CL (0.56 L/hr/kg) of cyclosporine in historical control subjects with normal renal function. In 5 liver transplant patients, the mean clearance of cyclosporine on and off hemodialysis was 463 mL/min and 398 mL/min, respectively. Less than 1% of the dose of cyclosporine was recovered in the dialysate.
Hepatic Impairment
Cyclosporine is extensively metabolized by the liver. Since severe hepatic impairment may result in significantly increased cyclosporine exposures, the dosage of cyclosporine may need to be reduced in these patients.
-
INDICATIONS AND USAGE
Sandimmune (cyclosporine) is indicated for the prophylaxis of organ rejection in kidney, liver, and heart allogeneic transplants. It is always to be used with adrenal corticosteroids. The drug may also be used in the treatment of chronic rejection in patients previously treated with other immunosuppressive agents.
Because of the risk of anaphylaxis, Sandimmune Injection (cyclosporine injection, USP) should be reserved for patients who are unable to take the soft gelatin capsules or oral solution.
- CONTRAINDICATIONS
-
WARNINGS
Kidney, Liver, and Heart Transplant
Sandimmune (cyclosporine), when used in high doses, can cause hepatotoxicity and nephrotoxicity (see BOXED WARNING).
Nephrotoxicity
It is not unusual for serum creatinine and BUN levels to be elevated during Sandimmune (cyclosporine) therapy. These elevations in renal transplant patients do not necessarily indicate rejection, and each patient must be fully evaluated before dosage adjustment is initiated.
Nephrotoxicity has been noted in 25% of cases of renal transplantation, 38% of cases of cardiac transplantation, and 37% of cases of liver transplantation. Mild nephrotoxicity was generally noted 2 to 3 months after transplant and consisted of an arrest in the fall of the preoperative elevations of BUN and creatinine at a range of 35 to 45 mg/dl and 2.0 to 2.5 mg/dl, respectively. These elevations were often responsive to dosage reduction.
More overt nephrotoxicity was seen early after transplantation and was characterized by a rapidly rising BUN and creatinine. Since these events are similar to rejection episodes, care must be taken to differentiate between them. This form of nephrotoxicity is usually responsive to Sandimmune (cyclosporine) dosage reduction.
Although specific diagnostic criteria which reliably differentiate renal graft rejection from drug toxicity have not been found, a number of parameters have been significantly associated to one or the other. It should be noted however, that up to 20% of patients may have simultaneous nephrotoxicity and rejection.
ap < 0.05, bp < 0.01, cp < 0.001, dp < 0.0001. Nephrotoxicity vs. Rejection Parameter Nephrotoxicity Rejection History Donor > 50 years old or hypotensive Antidonor immune response Prolonged kidney preservation Retransplant patient Prolonged anastomosis time Concomitant nephrotoxic drugs Clinical Often > 6 weeks postopb Often < 4 weeks postopb Prolonged initial nonfunction
(acute tubular necrosis)Fever > 37.5°C Weight gain > 0.5 kg Graft swelling and tenderness Decrease in daily urine volume > 500 mL (or 50%) Laboratory CyA serum trough level > 200 ng/mL CyA serum trough level < 150 ng/mL Gradual rise in Cr (< 0.15 mg/dL/day)a Rapid rise in Cr (> 0.3 mg/dL/day)a Cr plateau < 25% above baseline Cr > 25% above baseline BUN/Cr ≥ 20 BUN/Cr < 20 Biopsy Arteriolopathy (medial hypertrophya,
hyalinosis, nodular deposits, intimal
thickening, endothelial vacuolization,
progressive scarring)Endovasculitisc (proliferationa,
intimal arteritisb, necrosis, sclerosis)Tubular atrophy, isometric vacuolization,
isolated calcificationsTubulitis with RBCb and WBCb casts,
some irregular vacuolizationMinimal edema Interstitial edemac and hemorrhageb Mild focal infiltratesc Diffuse moderate to severe mononuclear infiltratesd Diffuse interstitial fibrosis,
often striped formGlomerulitis (mononuclear cells)c Aspiration Cytology CyA deposits in tubular and
endothelial cellsInflammatory infiltrate with mononuclear phagocytes,
macrophages, lymphoblastoid cells, and
activated T-cellsFine isometric vacuolization of
tubular cellsThese strongly express HLA-DR antigens Urine Cytology Tubular cells with vacuolization and
granularizationDegenerative tubular cells, plasma cells, and
lymphocyturia > 20% of sedimentManometry Intracapsular pressure < 40 mm Hgb Intracapsular pressure > 40 mm Hgb Ultrasonography Unchanged graft cross-sectional area Increase in graft cross-sectional area AP diameter ≥ Transverse diameter Magnetic Resonance
ImageryNormal appearance Loss of distinct corticomedullary junction, swelling,
image intensity of parachyma approaching that
of psoas, loss of hilar fatRadionuclide Scan Normal or generally decreased perfusion Patchy arterial flow Decrease in tubular function Decrease in perfusion > decrease in tubular function (131 I-hippuran) > decrease in perfusion
(99m Tc DTPA)Increased uptake of Indium 111 labeled platelets or
Tc-99m in colloidTherapy Responds to decreased Sandimmune
(cyclosporine)Responds to increased steroids or
antilymphocyte globulinA form of chronic progressive cyclosporine-associated nephrotoxicity is characterized by serial deterioration in renal function and morphologic changes in the kidneys. From 5% to 15% of transplant recipients will fail to show a reduction in a rising serum creatinine despite a decrease or discontinuation of cyclosporine therapy. Renal biopsies from these patients will demonstrate an interstitial fibrosis with tubular atrophy. In addition, toxic tubulopathy, peritubular capillary congestion, arteriolopathy, and a striped form of interstitial fibrosis with tubular atrophy may be present. Though none of these morphologic changes is entirely specific, a histologic diagnosis of chronic progressive cyclosporine-associated nephrotoxicity requires evidence of these.
When considering the development of chronic nephrotoxicity, it is noteworthy that several authors have reported an association between the appearance of interstitial fibrosis and higher cumulative doses or persistently high circulating trough concentrations of cyclosporine. This is particularly true during the first 6 posttransplant months when the dosage tends to be highest and when, in kidney recipients, the organ appears to be most vulnerable to the toxic effects of cyclosporine. Among other contributing factors to the development of interstitial fibrosis in these patients must be included, prolonged perfusion time, warm ischemia time, as well as episodes of acute toxicity, and acute and chronic rejection. The reversibility of interstitial fibrosis and its correlation to renal function have not yet been determined.
Impaired renal function at any time requires close monitoring, and frequent dosage adjustment may be indicated. In patients with persistent high elevations of BUN and creatinine who are unresponsive to dosage adjustments, consideration should be given to switching to other immunosuppressive therapy. In the event of severe and unremitting rejection, it is preferable to allow the kidney transplant to be rejected and removed rather than increase the Sandimmune (cyclosporine) dosage to a very high level in an attempt to reverse the rejection.
Due to the potential for additive or synergistic impairment of renal function, caution should be exercised when coadministering Sandimmune with other drugs that may impair renal function (see PRECAUTIONS, Drug Interactions).
Thrombotic Microangiopathy
Occasionally patients have developed a syndrome of thrombocytopenia and microangiopathic hemolytic anemia which may result in graft failure. The vasculopathy can occur in the absence of rejection and is accompanied by avid platelet consumption within the graft as demonstrated by Indium 111 labeled platelet studies. Neither the pathogenesis nor the management of this syndrome is clear. Though resolution has occurred after reduction or discontinuation of Sandimmune (cyclosporine) and 1) administration of streptokinase and heparin or 2) plasmapheresis, this appears to depend upon early detection with Indium 111 labeled platelet scans (see ADVERSE REACTIONS).
Hyperkalemia
Significant hyperkalemia (sometimes associated with hyperchloremic metabolic acidosis) and hyperuricemia have been seen occasionally in individual patients.
Hepatotoxicity
Cases of hepatotoxicity and liver injury, including cholestasis, jaundice, hepatitis, and liver failure have been reported in patients treated with cyclosporine. Most reports included patients with significant co-morbidities, underlying conditions and other confounding factors, including infectious complications and comedications with hepatotoxic potential. In some cases, mainly in transplant patients, fatal outcomes have been reported (see ADVERSE REACTIONS, Postmarketing Experience).
Hepatotoxicity, usually manifested by elevations in hepatic enzymes and bilirubin, was reported in patients treated with cyclosporine in clinical trials: 4% in renal transplantation, 7% in cardiac transplantation, and 4% in liver transplantation. This was usually noted during the first month of therapy when high doses of Sandimmune (cyclosporine) were used. The chemistry elevations usually decreased with a reduction in dosage.
Malignancies
As in patients receiving other immunosuppressants, those patients receiving Sandimmune (cyclosporine) are at increased risk for development of lymphomas and other malignancies, particularly those of the skin. The increased risk appears related to the intensity and duration of immunosuppression rather than to the use of specific agents. Because of the danger of oversuppression of the immune system, which can also increase susceptibility to infection, Sandimmune (cyclosporine) should not be administered with other immunosuppressive agents except adrenal corticosteroids. The efficacy and safety of cyclosporine in combination with other immunosuppressive agents have not been determined. Some malignancies may be fatal. Transplant patients receiving cyclosporine are at increased risk for serious infection with fatal outcome.
Serious Infections
Patients receiving immunosuppressants, including Sandimmune, are at increased risk of developing bacterial, viral, fungal, and protozoal infections, including opportunistic infections. These infections may lead to serious, including fatal, outcomes (see BOXED WARNING and ADVERSE REACTIONS).
Polyoma Virus Infections
Patients receiving immunosuppressants, including Sandimmune, are at increased risk for opportunistic infections, including polyoma virus infections. Polyoma virus infections in transplant patients may have serious, and sometimes, fatal outcomes. These include cases of JC virus-associated progressive multifocal leukoencephalopathy (PML), and polyoma virus-associated nephropathy (PVAN), especially due to BK virus infection, which have been observed in patients receiving cyclosporine.
PVAN is associated with serious outcomes, including deteriorating renal function and renal graft loss, (see ADVERSE REACTIONS, Postmarketing Experience). Patient monitoring may help detect patients at risk for PVAN.
Cases of PML have been reported in patients treated with Sandimmune. PML, which is sometimes fatal, commonly presents with hemiparesis, apathy, confusion, cognitive deficiencies, and ataxia. Risk factors for PML include treatment with immunosuppressant therapies and impairment of immune function. In immunosuppressed patients, physicians should consider PML in the differential diagnosis in patients reporting neurological symptoms and consultation with a neurologist should be considered as clinically indicated.
Consideration should be given to reducing the total immunosuppression in transplant patients who develop PML or PVAN. However, reduced immunosuppression may place the graft at risk.
Neurotoxicity
There have been reports of convulsions in adult and pediatric patients receiving cyclosporine, particularly in combination with high-dose methylprednisolone.
Encephalopathy, including Posterior Reversible Encephalopathy Syndrome (PRES), has been described both in postmarketing reports and in the literature. Manifestations include impaired consciousness, convulsions, visual disturbances (including blindness), loss of motor function, movement disorders, and psychiatric disturbances. In many cases, changes in the white matter have been detected using imaging techniques and pathologic specimens. Predisposing factors such as hypertension, hypomagnesemia, hypocholesterolemia, high-dose corticosteroids, high cyclosporine blood concentrations, and graft-versus-host disease have been noted in many but not all of the reported cases. The changes in most cases have been reversible upon discontinuation of cyclosporine, and in some cases, improvement was noted after reduction of dose. It appears that patients receiving liver transplant are more susceptible to encephalopathy than those receiving kidney transplant. Another rare manifestation of cyclosporine-induced neurotoxicity is optic disc edema, including papilloedema, with possible visual impairment, secondary to benign intracranial hypertension.
Specific Excipients
Anaphylactic Reactions
Rarely (approximately 1 in 1000), patients receiving Sandimmune Injection (cyclosporine injection, USP) have experienced anaphylactic reactions. Although the exact cause of these reactions is unknown, it is believed to be due to the Cremophor EL (polyoxyethylated castor oil) used as the vehicle for the intravenous (IV) formulation. These reactions can consist of flushing of the face and upper thorax, and noncardiogenic pulmonary edema, with acute respiratory distress, dyspnea, wheezing, blood pressure changes, and tachycardia. One patient died after respiratory arrest and aspiration pneumonia. In some cases, the reaction subsided after the infusion was stopped.
Patients receiving Sandimmune Injection (cyclosporine injection, USP) should be under continuous observation for at least the first 30 minutes following the start of the infusion and at frequent intervals thereafter. If anaphylaxis occurs, the infusion should be stopped. An aqueous solution of epinephrine 1:1000 should be available at the bedside as well as a source of oxygen.
Anaphylactic reactions have not been reported with the soft gelatin capsules or oral solution which lack Cremophor EL (polyoxyethylated castor oil). In fact, patients experiencing anaphylactic reactions have been treated subsequently with the soft gelatin capsules or oral solution without incident.
Alcohol (ethanol)
The alcohol content (see DESCRIPTION) of Sandimmune should be taken into account when given to patients in whom alcohol intake should be avoided or minimized, e.g., pregnant or breastfeeding women, in patients presenting with liver disease or epilepsy, in alcoholic patients, or pediatric patients. For an adult weighing 70 kg, the maximum daily oral dose would deliver about 1 gram of alcohol (see DESCRIPTION for alcohol content of each formulation).
Care should be taken in using Sandimmune (cyclosporine) with nephrotoxic drugs (see PRECAUTIONS).
Conversion from Neoral to Sandimmune
Because Sandimmune (cyclosporine) is not bioequivalent to Neoral, conversion from Neoral to Sandimmune (cyclosporine) using a 1:1 ratio (mg/kg/day) may result in a lower cyclosporine blood concentration. Conversion from Neoral to Sandimmune (cyclosporine) should be made with increased blood concentration monitoring to avoid the potential of underdosing.
-
PRECAUTIONS
General
Patients with malabsorption may have difficulty in achieving therapeutic concentrations with Sandimmune Soft Gelatin Capsules or Oral Solution.
Hypertension
Hypertension is a common side effect of Sandimmune (cyclosporine) therapy (see ADVERSE REACTIONS). Mild or moderate hypertension is more frequently encountered than severe hypertension and the incidence decreases over time. Antihypertensive therapy may be required. Control of blood pressure can be accomplished with any of the common antihypertensive agents. However, since cyclosporine may cause hyperkalemia, potassium-sparing diuretics should not be used. While calcium antagonists can be effective agents in treating cyclosporine-associated hypertension, care should be taken since interference with cyclosporine metabolism may require a dosage adjustment (see Drug Interactions).
Vaccination
During treatment with Sandimmune (cyclosporine), vaccination may be less effective and the use of live attenuated vaccines should be avoided.
-
Information for Patients
Patients should be advised that any change of cyclosporine formulation should be made cautiously and only under physician supervision because it may result in the need for a change in dosage.
Patients should be informed of the necessity of repeated laboratory tests while they are receiving the drug. They should be given careful dosage instructions, advised of the potential risks during pregnancy, and informed of the increased risk of neoplasia.
Patients using cyclosporine oral solution with its accompanying syringe for dosage measurement should be cautioned not to rinse the syringe either before or after use. Introduction of water into the product by any means will cause variation in dose.
Cyclosporine may impact the ability to drive and use machines. Patients should be advised to exercise care when driving or using machines if they experience neurological disturbances including confusion, somnolence, or dizziness and discuss with their healthcare provider (see WARNINGS and ADVERSE REACTIONS).
- Laboratory Tests
-
Drug Interactions
A. Effect of Drugs and Other Agents on Cyclosporine Pharmacokinetics and/or Safety
All of the individual drugs cited below are well substantiated to interact with cyclosporine. In addition, concomitant use of nonsteroidal anti-inflammatory drugs (NSAIDs) with cyclosporine, particularly in the setting of dehydration, may potentiate renal dysfunction. Caution should be exercised when using other drugs which are known to impair renal function (see WARNINGS, Nephrotoxicity).
Drugs That May Potentiate Renal Dysfunction
Antibiotics Antineoplastic Antifungals Anti-Inflammatory Drugs Gastrointestinal Agents Immunosuppressives Other Drugs ciprofloxacin melphalan amphotericin B azapropazon cimetidine tacrolimus fibric acid derivatives
(e.g., bezafibrate, fenofibrate)gentamicin ketoconazole colchicine ranitidine methotrexate tobramycin diclofenac trimethoprim
with sulfamethoxazolenaproxen vancomycin sulindac During the concomitant use of a drug that may exhibit additive or synergistic renal impairment potential with cyclosporine, close monitoring of renal function (in particular serum creatinine) should be performed. If a significant impairment of renal function occurs, reduction in the dosage of cyclosporine and/or coadministered drug or an alternative treatment should be considered.
Cyclosporine is extensively metabolized by CYP 3A isoenzymes, in particular CYP3A4, and is a substrate of the multidrug efflux transporter P-glycoprotein. Various agents are known to either increase or decrease plasma or whole blood concentrations of cyclosporine usually by inhibition or induction of CYP3A4 or P-glycoprotein transporter or both. Compounds that decrease cyclosporine absorption, such as orlistat, should be avoided. Appropriate Sandimmune (cyclosporine) dosage adjustment to achieve the desired cyclosporine concentrations is essential when drugs that significantly alter cyclosporine concentrations are used concomitantly (see DOSAGE AND ADMINISTRATION, Blood Concentration Monitoring).
1. Drugs That Increase Cyclosporine Concentrations
Calcium Channel Blockers Antifungals Antibiotics Glucocorticoids Other Drugs diltiazem fluconazole azithromycin methylprednisolone allopurinol nicardipine itraconazole clarithromycin amiodarone verapamil ketoconazole erythromycin bromocriptine voriconazole quinupristin/
dalfopristincolchicine danazol imatinib metoclopramide nefazodone oral contraceptives HIV Protease inhibitors
The HIV protease inhibitors (e.g., indinavir, nelfinavir, ritonavir, and saquinavir) are known to inhibit cytochrome P-450 3A and thus could potentially increase the concentrations of cyclosporine, however, no formal studies of the interaction are available. Care should be exercised when these drugs are administered concomitantly.
Grapefruit Juice
Grapefruit and grapefruit juice affect metabolism, increasing blood concentrations of cyclosporine, thus should be avoided.
2. Drugs/Dietary Supplements That Decrease Cyclosporine Concentrations
Antibiotics Anticonvulsants Other Drugs / Dietary Supplements nafcillin carbamazepine bosentan St. John’s Wort rifampin oxcarbazepine octreotide phenobarbital orlistat phenytoin sulfinpyrazone terbinafine ticlopidine Bosentan
Coadministration of bosentan (250 to 1000 mg every 12 hours based on tolerability) and cyclosporine (300 mg every 12 hours for 2 days then dosing to achieve a Cmin of 200 to 250 ng/mL) for 7 days in healthy subjects resulted in decreases in the cyclosporine mean dose-normalized AUC, Cmax, and trough concentration of approximately 50%, 30% and 60%, respectively, compared to when cyclosporine was given alone (see also Effect of Cyclosporine on the Pharmacokinetics and/or Safety of Other Drugs or Agents). Coadministration of cyclosporine with bosentan should be avoided.
Boceprevir
Coadministration of boceprevir (800 mg three times daily for 7 days) and cyclosporine (100 mg single dose) in healthy subjects resulted in increases in the mean AUC and Cmax of cyclosporine approximately 2.7-fold and 2-fold, respectively, compared to when cyclosporine was given alone.
Telaprevir
Coadministration of telaprevir (750 mg every 8 hours for 11 days) with cyclosporine (10 mg on Day 8) in healthy subjects resulted in increases in the mean dose-normalized AUC and Cmax of cyclosporine approximately 4.5-fold and 1.3-fold, respectively, compared to when cyclosporine (100 mg single dose) was given alone.
St. John’s Wort
There have been reports of a serious drug interaction between cyclosporine and the herbal dietary supplement, St. John’s Wort. This interaction has been reported to produce a marked reduction in the blood concentrations of cyclosporine, resulting in subtherapeutic levels, rejection of transplanted organs, and graft loss.
Rifabutin
Rifabutin is known to increase the metabolism of other drugs metabolized by the cytochrome P-450 system. The interaction between rifabutin and cyclosporine has not been studied. Care should be exercised when these two drugs are administered concomitantly.
B. Effect of Cyclosporine on the Pharmacokinetics and/or Safety of Other Drugs or Agents
Cyclosporine is an inhibitor of CYP3A4 and of multiple drug efflux transporters (e.g., P-glycoprotein) and may increase plasma concentrations of comedications that are substrates of CYP3A4, P-glycoprotein, or organic anion transporter proteins.
Cyclosporine may reduce the clearance of digoxin, colchicine, prednisolone, HMG-CoA reductase inhibitors (statins) and aliskiren, bosentan, dabigatran, repaglinide, NSAIDs, sirolimus, etoposide, and other drugs.
See the full prescribing information of the other drug for further information and specific recommendations. The decision on coadministration of cyclosporine with other drugs or agents should be made by the healthcare provider following the careful assessment of benefits and risks.
Digoxin
Severe digitalis toxicity has been seen within days of starting cyclosporine in several patients taking digoxin. If digoxin is used concurrently with cyclosporine, serum digoxin concentrations should be monitored.
Colchicine
There are reports on the potential of cyclosporine to enhance the toxic effects of colchicine, such as myopathy and neuropathy, especially in patients with renal dysfunction. Concomitant administration of cyclosporine and colchicine results in significant increases in colchicine plasma concentrations. If colchicine is used concurrently with cyclosporine, a reduction in the dosage of colchicine is recommended.
HMG Co-A Reductase Inhibitors (Statins)
Literature and postmarketing cases of myotoxicity, including muscle pain and weakness, myositis, and rhabdomyolysis, have been reported with concomitant administration of cyclosporine with lovastatin, simvastatin, atorvastatin, pravastatin, and rarely, fluvastatin. When concurrently administered with cyclosporine, the dosage of these statins should be reduced according to label recommendations. Statin therapy needs to be temporarily withheld or discontinued in patients with signs and symptoms of myopathy or those with risk factors predisposing to severe renal injury, including renal failure, secondary to rhabdomyolysis.
Repaglinide
Cyclosporine may increase the plasma concentrations of repaglinide and thereby increase the risk of hypoglycemia. In 12 healthy male subjects who received two doses of 100 mg cyclosporine capsule orally 12 hours apart with a single dose of 0.25 mg repaglinide tablet (one half of a 0.5 mg tablet) orally 13 hours after the cyclosporine initial dose, the repaglinide mean Cmax and AUC were increased 1.8-fold (range, 0.6 to 3.7-fold) and 2.4-fold (range, 1.2 to 5.3-fold), respectively. Close monitoring of blood glucose level is advisable for a patient taking cyclosporine and repaglinide concomitantly.
Ambrisentan
Coadministration of ambrisentan (5 mg daily) and cyclosporine (100 to 150 mg twice daily initially, then dosing to achieve Cmin 150 to 200 ng/mL) for 8 days in healthy subjects resulted mean increases in ambrisentan AUC and Cmax of approximately 2-fold and 1.5-fold, respectively, compared to ambrisentan alone. When coadministering ambrisentan with cyclosporine, the ambrisentan dose should not be titrated to the recommended maximum daily dose.
Anthracycline Antibiotics
High doses of cyclosporine (e.g., at starting intravenous dose of 16 mg/kg/day) may increase the exposure to anthracycline antibiotics (e.g., doxorubicin, mitoxantrone, daunorubicin) in cancer patients.
Aliskiren
Cyclosporine alters the pharmacokinetics of aliskiren, a substrate of P-glycoprotein and CYP3A4. In 14 healthy subjects who received concomitantly single doses of cyclosporine (200 mg) and reduced dose aliskiren (75 mg), the mean Cmax of aliskiren was increased by approximately 2.5-fold (90% CI: 1.96 to 3.17) and the mean AUC by approximately 4.3-fold (90% CI: 3.52 to 5.21), compared to when these subjects received aliskiren alone. The concomitant administration of aliskiren with cyclosporine prolonged the median aliskiren elimination half-life (26 hours versus 43 to 45 hours) and the Tmax (0.5 hours versus 1.5 to 2.0 hours). The mean AUC and Cmax of cyclosporine were comparable to reported literature values. Coadministration of cyclosporine and aliskiren in these subjects also resulted in an increase in the number and/or intensity of adverse events, mainly headache, hot flush, nausea, vomiting, and somnolence. The coadministration of cyclosporine with aliskiren is not recommended.
Bosentan
In healthy subjects, coadministration of bosentan and cyclosporine resulted in time-dependent mean increases in dose-normalized bosentan trough concentrations (i.e., approximately 21-fold on Day 1 and 2-fold on Day 8 (steady state)) compared to when bosentan was given alone as a single dose on Day 1 (see also Effect of Drugs and Other Agents on Cyclosporine Pharmacokinetics and/or Safety). Coadministration of cyclosporine with bosentan should be avoided.
Dabigatran
The effect of cyclosporine on dabigatran concentrations had not been formally studied. Concomitant administration of dabigatran and cyclosporine may result in increased plasma dabigatran concentrations due to the P-gp inhibitory activity of cyclosporine. Coadministration of cyclosporine with dabigatran should be avoided.
Potassium Sparing Diuretics
Cyclosporine should not be used with potassium-sparing diuretics because hyperkalemia can occur. Caution is also required when cyclosporine is coadministered with potassium-sparing drugs (e.g., angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists), potassium-containing drugs as well as in patients on a potassium-rich diet. Control of potassium levels in these situations is advisable.
Nonsteroidal Anti-inflammatory Drug (NSAID) Interactions
Clinical status and serum creatinine should be closely monitored when cyclosporine is used with NSAIDs in rheumatoid arthritis patients (see WARNINGS).
Pharmacodynamic interactions have been reported to occur between cyclosporine and both naproxen and sulindac, in that concomitant use is associated with additive decreases in renal function, as determined by 99mTc-diethylenetriaminepenta acetic acid (DTPA) and (p-aminohippuric acid) PAH clearances. Although concomitant administration of diclofenac does not affect blood concentrations of cyclosporine, it has been associated with approximate doubling of diclofenac blood levels and occasional reports of reversible decreases in renal function. Consequently, the dose of diclofenac should be in the lower end of the therapeutic range.
Methotrexate Interaction
Preliminary data indicate that when methotrexate and cyclosporine were coadministered to rheumatoid arthritis patients (N = 20), methotrexate concentrations (AUCs) were increased approximately 30% and the concentrations (AUCs) of its metabolite, 7-hydroxy methotrexate, were decreased by approximately 80%. The clinical significance of this interaction is not known. Cyclosporine concentrations do not appear to have been altered (N = 6).
Sirolimus
Elevations in serum creatinine were observed in studies using sirolimus in combination with full-dose cyclosporine. This effect is often reversible with cyclosporine dose reduction. Simultaneous coadministration of cyclosporine significantly increases blood levels of sirolimus. To minimize increases in sirolimus blood concentrations, it is recommended that sirolimus be given 4 hours after cyclosporine administration.
Nifedipine
Frequent gingival hyperplasia when nifedipine is given concurrently with cyclosporine has been reported. The concomitant use of nifedipine should be avoided in patients in whom gingival hyperplasia develops as a side effect of cyclosporine.
Methylprednisolone
Convulsions when high dose methylprednisolone is given concomitantly with cyclosporine have been reported.
Other Immunosuppressive Drugs and Agents
Psoriasis patients receiving other immunosuppressive agents or radiation therapy (including PUVA and UVB) should not receive concurrent cyclosporine because of the possibility of excessive immunosuppression.
Interactions Resulting in Decrease of Other Drug Levels
Cyclosporine inhibits the enterohepatic circulation of mycophenolic acid (MPA). Concomitant administration of cyclosporine and mycophenolate mofetil or mycophenolate sodium in transplant patients may decrease the mean exposure of MPA by 20% - 50% when compared with other immunosuppressants, which could reduce efficacy of mycophenolate mofetil or mycophenolate sodium. Monitor patients for alterations in efficacy of mycophenolate mofetil or mycophenolate sodium, when they are coadministered with cyclosporine.
C. Effect of Cyclosporine on the Efficacy of Live Vaccines
During treatment with cyclosporine, vaccination may be less effective. The use of live vaccines should be avoided.
For additional information on Cyclosporine Drug Interactions please contact Novartis Medical Affairs Department at 1-888-NOW-NOVA (1-888-669-6682).
-
Carcinogenesis, Mutagenesis, and Impairment of Fertility
Cyclosporine gave no evidence of mutagenic or teratogenic effects in appropriate test systems. Only at dose levels toxic to dams, were adverse effects seen in reproduction studies in rats (see Pregnancy).
Carcinogenicity studies were carried out in male and female rats and mice. In the 78-week mouse study, at doses of 1, 4, and 16 mg/kg/day, evidence of a statistically significant trend was found for lymphocytic lymphomas in females, and the incidence of hepatocellular carcinomas in mid-dose (0.03 times the maximum recommended human dose (MRHD) based on body surface area (BSA) males significantly exceeded the control value. In the 24–month rat study, conducted at 0.5, 2, and 8 mg/kg/day, pancreatic islet cell adenomas significantly exceeded the control rate in the low-dose level (0.006 times the MRHD based on BSA). The hepatocellular carcinomas and pancreatic islet cell adenomas were not dose related.
Cyclosporine has not been found mutagenic/genotoxic in the Ames test, the V79-HGPRT Test, the micronucleus test in mice and Chinese hamsters, the chromosome-aberration tests in Chinese hamster bone marrow, the mouse dominant lethal assay, and the DNA-repair test in sperm from treated mice. A recent study analyzing sister chromatid exchange (SCE) induction by cyclosporine using human lymphocytes in vitro gave indication of a positive effect (i.e., induction of SCE), at high concentrations in this system.
In a fertility study in rats, increased perinatal mortality and impaired postnatal development of F1 pups were observed at 15 mg/kg/day (0.2 times the MRHD based on BSA). No adverse effects on fertility and reproduction were observed up to 5 mg/kg/day (0.06 times the MRHD based on BSA) in male and female rats.
An increased incidence of malignancy is a recognized complication of immunosuppression in recipients of organ transplants. The most common forms of neoplasms are non-Hodgkin’s lymphoma and carcinomas of the skin. The risk of malignancies in cyclosporine recipients is higher than in the normal, healthy population, but similar to that in patients receiving other immunosuppressive therapies. It has been reported that reduction or discontinuance of immunosuppression may cause the lesions to regress.
-
Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to cyclosporine, including Sandimmune, during pregnancy. Encourage women who are taking Sandimmune during pregnancy to enroll in the Transplant Pregnancy Registry International (TPRI) by calling 1-877-955-8677 or visiting https://www.transplantpregnancyregistry.org.
Risk Summary
Available data from published literature, including the Transplant Pregnancy Registry International, observational cohort studies, case-controlled studies, meta-analysis, case series, and case reports, over decades of use with cyclosporine in pregnancy have not identified a drug associated risk of major birth defects, or miscarriage. Adverse maternal or fetal outcomes including hypertension, preeclampsia, preterm birth, and low birth weight are increased in patients treated with cyclosporine. However, patients receiving cyclosporine during pregnancy have underlying medical conditions and may be treated with concomitant medications that limit the interpretability of these findings (see Data).
Embryo-fetal developmental (EFD) studies in rats and rabbits with cyclosporine have shown embryo-fetal toxicity at dose levels below the MRHD based on BSA.
The alcohol content of Sandimmune should be taken into account when given to pregnant women (see WARNINGS, Special Excipients).
The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Human Data
Available data from the National Transplantation Pregnancy Registry (NTPR) including 622 pregnancies in renal, liver, and heart transplant recipients exposed to cyclosporine during pregnancy found that the overall rate of major birth defects, live birth rates, and miscarriage rates were comparable to the general population. Maternal and fetal adverse outcomes, including the rate of hypertension, preeclampsia, premature births, and low birth weight infants appear to be increased in transplant recipients treated with cyclosporine compared to the general population. However, these patients have underlying medical conditions that confound the above findings.
Animal Data
Animal studies have shown reproductive toxicity in rats and rabbits.
Three EFD studies (two oral and one intravenous) are available in rats. In two EFD studies, pregnant rats were orally administered with cyclosporine either at doses of 10, 17, 30, 100 and 300 mg/kg/day or 4, 10 and 25 mg/kg/day from gestation day (GD) 6 to 15 or from GD 7 to 17, respectively. Maternal toxicity characterized by mortality, clinical signs of toxicity and impaired body weight gain were observed at 30 mg/kg/day and above. Cyclosporine was embryo- and fetotoxic as indicated by increased embryonic mortality and reduced fetal weight together with skeletal retardations in rats at 25 mg/kg/day and above. In addition, ventricular septal defect was observed at 25 mg/kg/day in fetuses. In the first study, the oral no observed effect level (NOEL) for both dams and fetuses was 17 mg/kg/day (0.2 times the MRHD based on BSA). In the other oral study, the NOEL for dams and fetuses were 10 and 4 mg/kg/day (0.13 and 0.05 times the MRHD based on BSA), respectively. In the IV EFD study, rats were administered with 3, 6 and 12 mg/kg/day of cyclosporine from GD 7 to 17. An increase in post implantation loss was observed at 12 mg/kg/day; ventricular septal defect was observed at ≥ 6 mg/kg/day in fetuses. The IV NOEL for dams and fetus were 6 and 3 mg/kg/day (below the MRHD based on BSA), respectively after IV administration.
In rabbits, cyclosporine was orally administered at dose levels of 10, 30, 100 or 300 mg/kg/day from GD 6 to 18. At 100 mg/kg/day and above, reduction in body weight gain of dams and at 300 mg/kg/day abortions were observed. Maternal toxicity, embryo-fetotoxicity as indicated by increased pre- and postnatal mortality, reduced fetal weight together with skeletal retardations were observed at 100 mg/kg/day and above. The NOEL for dams and fetuses was 30 mg/kg/day (1 times the MRHD based on BSA).
In two published research studies, rabbits exposed to cyclosporine in utero (10 mg/kg/day subcutaneously) demonstrated reduced numbers of nephrons, renal hypertrophy, systemic hypertension and progressive renal insufficiency up to 35 weeks of age. These findings have not been demonstrated in other species and their relevance for humans is unknown.
In a peri- and postnatal development study in rats, pregnant rats were orally administered with cyclosporine (5, 15 or 45 mg/kg/day) from GD 15 until end of lactation. At 45 mg/kg/day (0.5 times the MRHD based on BSA), increased pre and postnatal mortality of offspring and reduced body weight gain of surviving pups were observed. Cyclosporine up to 15 mg/kg/day (0.2 times the MRHD based on BSA) had no effect on pregnancy, pre and postnatal development of offspring.
-
Nursing Mothers
Cyclosporine and its metabolites are present in human milk following oral and intravenous administration. Adverse effects on the breastfed infant have not been reported. There are no data on the effects of the drug on milk production. The alcohol content of Sandimmune should be taken into account when given to lactating women (see WARNINGS, Special Excipients). Lactating women are encouraged to avoid additional alcohol intake during treatment. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Sandimmune and any potential adverse effects on the breastfed infant from Sandimmune or from the underlying maternal condition.
- Pediatric Use
-
Geriatric Use
Clinical studies of Sandimmune (cyclosporine) did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger patients. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
ADVERSE REACTIONS
The principal adverse reactions of Sandimmune (cyclosporine) therapy are renal dysfunction, tremor, hirsutism, hypertension, and gum hyperplasia.
Hypertension
Hypertension, which is usually mild to moderate, may occur in approximately 50% of patients following renal transplantation and in most cardiac transplant patients.
Glomerular Capillary Thrombosis
Glomerular capillary thrombosis has been found in patients treated with cyclosporine and may progress to graft failure. The pathologic changes resemble those seen in the hemolytic-uremic syndrome and include thrombosis of the renal microvasculature, with platelet-fibrin thrombi occluding glomerular capillaries and afferent arterioles, microangiopathic hemolytic anemia, thrombocytopenia, and decreased renal function. Similar findings have been observed when other immunosuppressives have been employed post transplantation.
Hypomagnesemia
Hypomagnesemia has been reported in some, but not all, patients exhibiting convulsions while on cyclosporine therapy. Although magnesium-depletion studies in normal subjects suggest that hypomagnesemia is associated with neurologic disorders, multiple factors, including hypertension, high-dose methylprednisolone, hypocholesterolemia, and nephrotoxicity associated with high plasma concentrations of cyclosporine appear to be related to the neurological manifestations of cyclosporine toxicity.
Clinical Studies
The following reactions occurred in 3% or greater of 892 patients involved in clinical trials of kidney, heart, and liver transplants:
Randomized Kidney Patients All Sandimmune (cyclosporine) Patients Sandimmune Azathioprine Kidney Heart Liver Body System/ (N = 227) (N = 228) (N = 705) (N = 112) (N = 75) Adverse Reactions % % % % % Genitourinary Renal Dysfunction 32 6 25 38 37 Cardiovascular Hypertension 26 18 13 53 27 Cramps 4 < 1 2 < 1 0 Skin Hirsutism 21 < 1 21 28 45 Acne 6 8 2 2 1 Central Nervous System Tremor 12 0 21 31 55 Convulsions 3 1 1 4 5 Headache 2 < 1 2 15 4 Gastrointestinal Gum Hyperplasia 4 0 9 5 16 Diarrhea 3 < 1 3 4 8 Nausea/Vomiting 2 < 1 4 10 4 Hepatotoxicity < 1 < 1 4 7 4 Abdominal Discomfort < 1 0 < 1 7 0 Autonomic Nervous System Paresthesia 3 0 1 2 1 Flushing < 1 0 4 0 4 Hematopoietic Leukopenia 2 19 < 1 6 0 Lymphoma < 1 0 1 6 1 Respiratory Sinusitis < 1 0 4 3 7 Miscellaneous Gynecomastia < 1 0 < 1 4 3 The following reactions occurred in 2% or less of patients: allergic reactions, anemia, anorexia, confusion, conjunctivitis, edema, fever, brittle fingernails, gastritis, hearing loss, hiccups, hyperglycemia, muscle pain, peptic ulcer, thrombocytopenia, tinnitus.
The following reactions occurred rarely: anxiety, chest pain, constipation, depression, hair breaking, hematuria, joint pain, lethargy, mouth sores, myocardial infarction, night sweats, pancreatitis, pruritus, swallowing difficulty, tingling, upper GI bleeding, visual disturbance, weakness, weight loss.
Sandimmune (cyclosporine) was discontinued on a temporary basis and then restarted in 18 additional patients. Renal Transplant Patients in Whom Therapy Was Discontinued Randomized Patients All Sandimmune Patients Sandimmune Azathioprine (N = 227) (N = 228) (N = 705) Reason for Discontinuation % % % Renal Toxicity 5.7 0 5.4 Infection 0 0.4 0.9 Lack of Efficacy 2.6 0.9 1.4 Acute Tubular Necrosis 2.6 0 1.0 Lymphoma/Lymphoproliferative Disease 0.4 0 0.3 Hypertension 0 0 0.3 Hematological Abnormalities 0 0.4 0 Other 0 0 0.7 Patients receiving immunosuppressive therapies, including cyclosporine and cyclosporine-containing regimens, are at increased risk of infections (viral, bacterial, fungal, and parasitic). Both generalized and localized infections can occur. Preexisting infections may also be aggravated. Fatal outcomes have been reported (see WARNINGS).
*Some patients also received ALG. Infectious Complications in the Randomized Renal Transplant Patients Sandimmune Treatment Standard Treatment* (N = 227) (N = 228) Complication % of Complications % of Complications Septicemia 5.3 4.8 Abscesses 4.4 5.3 Systemic Fungal Infection 2.2 3.9 Local Fungal Infection 7.5 9.6 Cytomegalovirus 4.8 12.3 Other Viral Infections 15.9 18.4 Urinary Tract Infections 21.1 20.2 Wound and Skin Infections 7.0 10.1 Pneumonia 6.2 9.2 Cremophor® EL (polyoxyethylated castor oil) is known to cause hyperlipemia and electrophoretic abnormalities of lipoproteins. These effects are reversible upon discontinuation of treatment but are usually not a reason to stop treatment.
Postmarketing Experience
Hepatotoxicity
Cases of hepatotoxicity and liver injury, including cholestasis, jaundice, hepatitis, and liver failure; serious and/or fatal outcomes have been reported (see WARNINGS, Hepatotoxicity).
Increased Risk of Infections
Cases of JC virus-associated progressive multifocal leukoencephalopathy (PML), sometimes fatal; and polyoma virus-associated nephropathy (PVAN), especially BK virus resulting in graft loss have been reported (see WARNINGS, Polyoma Virus Infection).
Headache, Including Migraine
Cases of migraine have been reported. In some cases, patients have been unable to continue cyclosporine, however, the final decision on treatment discontinuation should be made by the treating physician following the careful assessment of benefits versus risks.
Pain of Lower Extremities
Isolated cases of pain of lower extremities have been reported in association with cyclosporine. Pain of lower extremities has also been noted as part of Calcineurin-Inhibitor Induced Pain Syndrome (CIPS) as described in the literature.
-
OVERDOSAGE
There is a minimal experience with overdosage. Because of the slow absorption of Sandimmune Soft Gelatin Capsules or Oral Solution, forced emesis and gastric lavage would be of value up to 2 hours after administration. Transient hepatotoxicity and nephrotoxicity may occur which should resolve following drug withdrawal. Oral doses of cyclosporine up to 10 g (about 150 mg/kg) have been tolerated with relatively minor clinical consequences, such as vomiting, drowsiness, headache, tachycardia, and in a few patients, moderately severe, reversible impairment of renal function. However, serious symptoms of intoxication have been reported following accidental parenteral overdosage with cyclosporine in premature neonates. General supportive measures and symptomatic treatment should be followed in all cases of overdosage. Sandimmune (cyclosporine) is not dialyzable to any great extent, nor is it cleared well by charcoal hemoperfusion. The oral LD50 is 2329 mg/kg in mice, 1480 mg/kg in rats, and > 1000 mg/kg in rabbits. The intravenous (IV) LD50 is 148 mg/kg in mice, 104 mg/kg in rats, and 46 mg/kg in rabbits.
-
DOSAGE AND ADMINISTRATION
Sandimmune Soft Gelatin Capsules (cyclosporine capsules, USP) and Sandimmune Oral Solution (cyclosporine oral solution, USP)
Sandimmune Soft Gelatin Capsules (cyclosporine capsules, USP) and Sandimmune Oral Solution (cyclosporine oral solution, USP) have decreased bioavailability in comparison to Neoral Soft Gelatin Capsules (cyclosporine capsules, USP) MODIFIED and Neoral Oral Solution (cyclosporine oral solution, USP) MODIFIED. Sandimmune and Neoral are not bioequivalent and cannot be used interchangeably without physician supervision.
The initial oral dose of Sandimmune (cyclosporine) should be given 4 to 12 hours prior to transplantation as a single dose of 15 mg/kg. Although a daily single dose of 14 to 18 mg/kg was used in most clinical trials, few centers continue to use the highest dose, most favoring the lower end of the scale. There is a trend towards use of even lower initial doses for renal transplantation in the ranges of 10 to 14 mg/kg/day. The initial single daily dose is continued postoperatively for 1 to 2 weeks and then tapered by 5% per week to a maintenance dose of 5 to 10 mg/kg/day. Some centers have successfully tapered the maintenance dose to as low as 3 mg/kg/day in selected renal transplant patients without an apparent rise in rejection rate.
See Blood Concentration Monitoring, below.
Specific Populations
Renal Impairment
Cyclosporine undergoes minimal renal elimination and its pharmacokinetics do not appear to be significantly altered in patients with end-stage renal disease who receive routine hemodialysis treatments (see CLINICAL PHARMACOLOGY). However, due to its nephrotoxic potential (see WARNINGS), careful monitoring of renal function is recommended; cyclosporine dosage should be reduced if indicated (see WARNINGS and PRECAUTIONS).
Hepatic Impairment
The clearance of cyclosporine may be significantly reduced in severe liver disease patients (see CLINICAL PHARMACOLOGY). Dose reduction may be necessary in patients with severe liver impairment to maintain blood concentrations within the recommended target range (see WARNINGS and PRECAUTIONS).
Pediatrics
In pediatric usage, the same dose and dosing regimen may be used as in adults although in several studies, children have required and tolerated higher doses than those used in adults.
Adjunct therapy with adrenal corticosteroids is recommended. Different tapering dosage schedules of prednisone appear to achieve similar results. A dosage schedule based on the patient’s weight started with 2.0 mg/kg/day for the first 4 days tapered to 1.0 mg/kg/day by 1 week, 0.6 mg/kg/day by 2 weeks, 0.3 mg/kg/day by 1 month, and 0.15 mg/kg/day by 2 months and thereafter as a maintenance dose. Another center started with an initial dose of 200 mg tapered by 40 mg/day until reaching 20 mg/day. After 2 months at this dose, a further reduction to 10 mg/day was made. Adjustments in dosage of prednisone must be made according to the clinical situation.
To make Sandimmune Oral Solution (cyclosporine oral solution, USP) more palatable, the oral solution may be diluted with milk, chocolate milk, or orange juice preferably at room temperature. Patients should avoid switching diluents frequently. Sandimmune Soft Gelatin Capsules and Oral Solution should be administered on a consistent schedule with regard to time of day and relation to meals.
Take the prescribed amount of Sandimmune (cyclosporine) from the container using the dosage syringe supplied after removal of the protective cover, and transfer the solution to a glass of milk, chocolate milk, or orange juice. Stir well and drink at once. Do not allow to stand before drinking. It is best to use a glass container and rinse it with more diluent to ensure that the total dose is taken. After use, replace the dosage syringe in the protective cover. Do not rinse the dosage syringe with water or other cleaning agents either before or after use. If the dosage syringe requires cleaning, it must be completely dry before resuming use. Introduction of water into the product by any means will cause variation in dose.
Sandimmune® Injection (cyclosporine injection, USP)
FOR INFUSION ONLY
Note: Anaphylactic reactions have occurred with Sandimmune Injection (cyclosporine injection, USP). See WARNINGS.
Patients unable to take Sandimmune Soft Gelatin Capsules or Oral Solution pre- or postoperatively may be treated with the intravenous (IV) concentrate. Sandimmune Injection (cyclosporine injection, USP) is administered at 1/3 the oral dose. The initial dose of Sandimmune Injection (cyclosporine injection, USP) should be given 4 to 12 hours prior to transplantation as a single intravenous dose of 5 to 6 mg/kg/day. This daily single dose is continued postoperatively until the patient can tolerate the soft gelatin capsules or oral solution. Patients should be switched to Sandimmune Soft Gelatin Capsules or Oral Solution as soon as possible after surgery. In pediatric usage, the same dose and dosing regimen may be used, although higher doses may be required.
Adjunct steroid therapy is to be used (See aforementioned).
Immediately before use, the intravenous concentrate should be diluted 1 mL Sandimmune Injection (cyclosporine injection, USP) in 20 mL to 100 mL 0.9% Sodium Chloride Injection or 5% Dextrose Injection and given in a slow intravenous infusion over approximately 2 to 6 hours.
Diluted infusion solutions should be discarded after 24 hours.
The Cremophor® EL (polyoxyethylated castor oil) contained in the concentrate for intravenous infusion can cause phthalate stripping from PVC.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Blood Concentration Monitoring
Several study centers have found blood concentration monitoring of cyclosporine useful in patient management. While no fixed relationships have yet been established, in one series of 375 consecutive cadaveric renal transplant recipients, dosage was adjusted to achieve specific whole blood 24-hour trough concentrations of 100 to 200 ng/mL as determined by high-pressure liquid chromatography (HPLC).
Of major importance to blood concentration analysis is the type of assay used. The above concentrations are specific to the parent cyclosporine molecule and correlate directly to the new monoclonal specific radioimmunoassays (mRIA-sp). Nonspecific assays are also available which detect the parent compound molecule and various of its metabolites. Older studies often cited concentrations using a nonspecific assay which were roughly twice those of specific assays. Assay results are not interchangeable and their use should be guided by their approved labeling. If plasma specimens are employed, concentrations will vary with the temperature at the time of separation from whole blood. Plasma concentrations may range from 1/2 to 1/5 of whole blood concentrations. Refer to individual assay labeling for complete instructions. In addition, Transplantation Proceedings (June 1990) contains position papers and a broad consensus generated at the Cyclosporine-Therapeutic Drug Monitoring conference that year. Blood concentration monitoring is not a replacement for renal function monitoring or tissue biopsies.
-
HOW SUPPLIED
Sandimmune® Soft Gelatin Capsules (cyclosporine capsules, USP)
25 mg: Oval, pink, branded “
 78/240”. Unit dose packages of 30 capsules,
78/240”. Unit dose packages of 30 capsules,3 blister cards of 10 capsules………………………………………………………….NDC 0078-0240-15
100 mg: Oblong, dusty rose, branded “
 78/241”. Unit dose packages of 30 capsules,
78/241”. Unit dose packages of 30 capsules,3 blister cards of 10 capsules………………………………………………………….NDC 0078-0241-15
Store and Dispense: Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature].
An odor may be detected upon opening the unit dose container, which will dissipate shortly thereafter. This odor does not affect the quality of the product.
Sandimmune® Oral Solution (cyclosporine oral solution, USP)
Supplied in 50 mL bottles containing 100 mg of cyclosporine per mL NDC 0078-0110-22
A dosage syringe is provided for dispensing.
Store and Dispense: In the original container at temperatures below 30°C (86°F). Do not store in the refrigerator. Protect from freezing. Once opened, the contents must be used within 2 months.
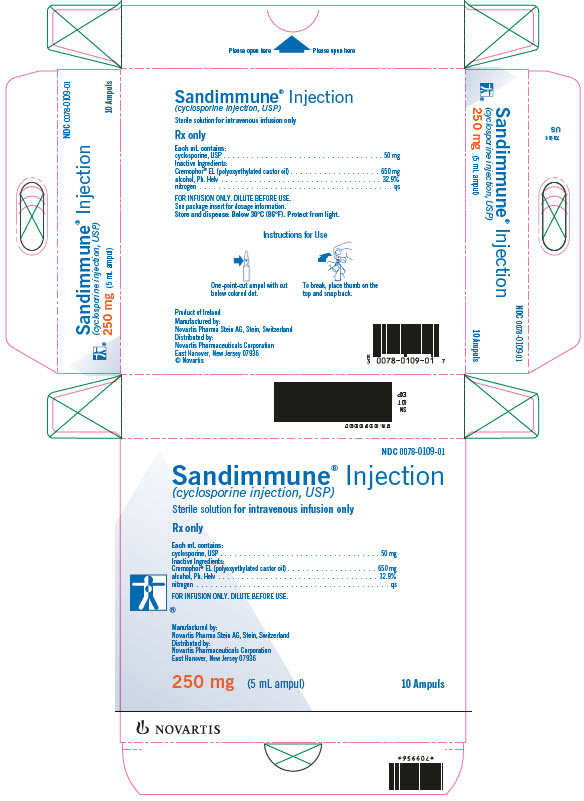
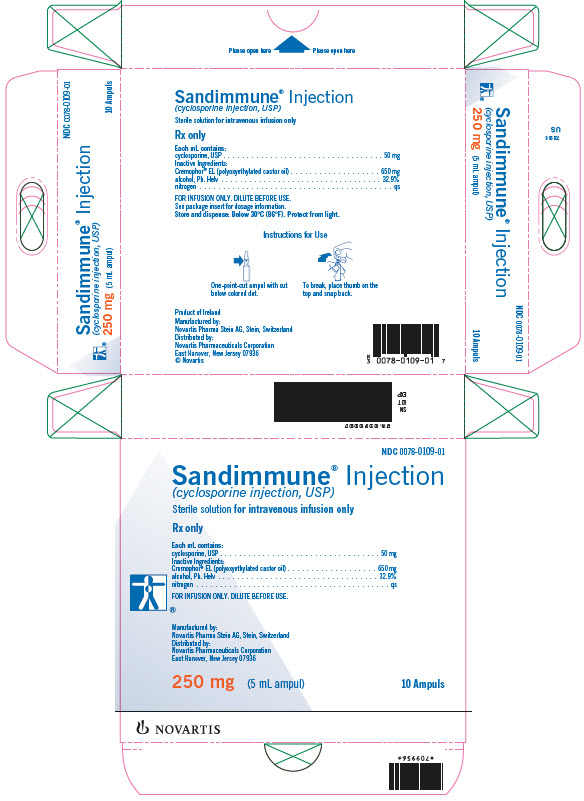
Sandimmune® Injection (cyclosporine injection, USP)
FOR INTRAVENOUS INFUSION
Supplied as a 5 mL sterile ampul containing 50 mg of cyclosporine per mL,
in boxes of 10 ampuls…………………………………………………………………..NDC 0078-0109-01
Store and Dispense: At temperatures below 30°C (86°F). Protect from light.
FOR INFUSION ONLY
*Cremophor® is the registered trademark of BASF Aktiengesellschaft.
Distributed by:
Novartis Pharmaceuticals Corporation
East Hanover, New Jersey 07936© Novartis
Revised: September 2023
T2023-72
-
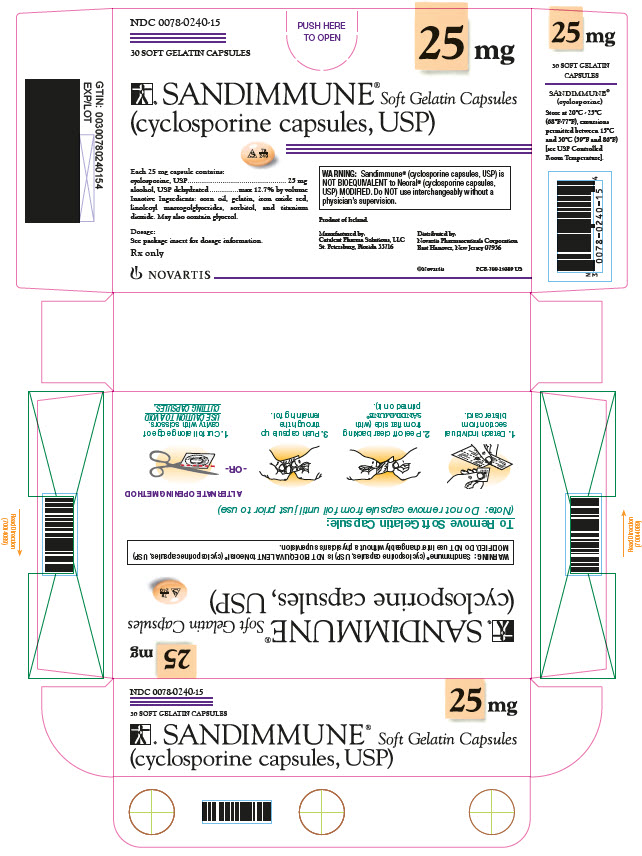
PRINCIPAL DISPLAY PANEL
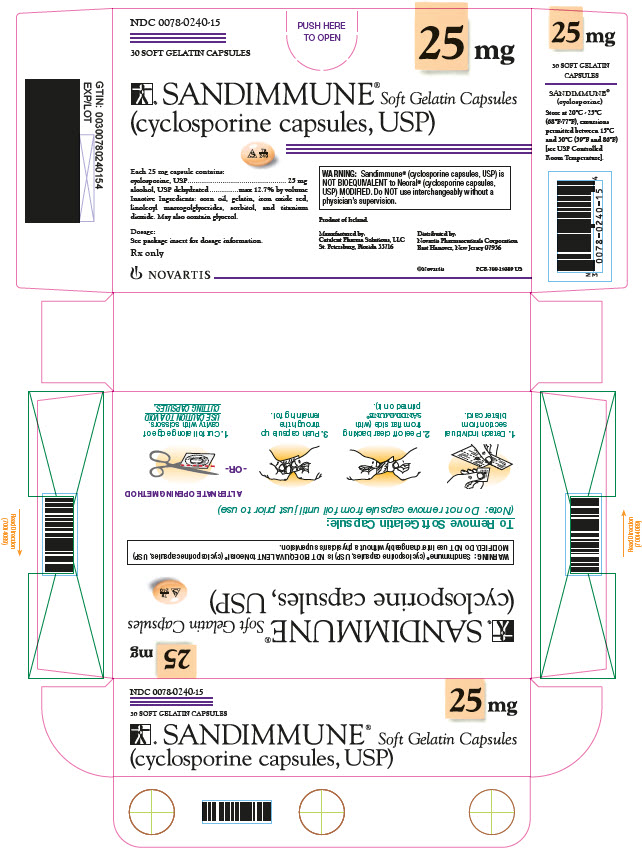
NDC 0078-0240-15
25 mg
30 SOFT GELATIN CAPSULES
SANDIMMUNE® Soft Gelatin Capsules
(cyclosporine capsules, USP)
Each 25 mg capsule contains:
cyclosporine, USP 25 mg
alcohol, USP dehydrated max 12.7% by volume
Inactive Ingredients: corn oil, gelatin, iron oxide red, linoleoyl macrogolglycerides, sorbitol, and titanium dioxide. May also contain glycerol.
WARNING: Sandimmune® (cyclosporine capsules, USP) is
NOT BIOEQUIVALENT to Neoral® (cyclosporine capsules, USP) MODIFIED.
Do NOT use interchangeably without a physician’s supervision.
Dosage:
See package insert for dosage information.
Rx only
NOVARTIS
-
PRINCIPAL DISPLAY PANEL
NDC 0078-0241-15
100 mg
30 SOFT GELATIN CAPSULES
SANDIMMUNE® Soft Gelatin Capsules
(cyclosporine capsules, USP)
Each 100 mg capsule contains:
cyclosporine, USP 100 mg
alcohol, USP dehydrated max 12.7% by volume
Inactive Ingredients: corn oil, gelatin, iron oxide red, linoleoyl macrogolglycerides, sorbitol, and titanium dioxide. May also contain glycerol or iron oxide yellow.
WARNING: Sandimmune® (cyclosporine capsules, USP) is
NOT BIOEQUIVALENT to Neoral® (cyclosporine capsules, USP) MODIFIED.
Do NOT use interchangeably without a physician’s supervision.
Dosage:
See package insert for dosage information.
Rx only
NOVARTIS

-
PRINCIPAL DISPLAY PANEL
NDC 0078-0110-22
50 mL
SANDIMMUNE® Oral Solution
(cyclosporine oral solution, USP)
100 mg/mL
WARNING: Sandimmune® (cyclosporine
oral solution, USP) is NOT BIOEQUIVALENT
to Neoral® (cyclosporine oral solution, USP)
MODIFIED. Do NOT use interchangeably
without a physician’s supervision.Each mL contains:
cyclosporine, USP 100 mg
dehydrated alcohol, Ph. Helv 12.5%
Rx only
NOVARTIS

- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SANDIMMUNE
cyclosporine capsule, liquid filledProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0078-0240 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CYCLOSPORINE (UNII: 83HN0GTJ6D) (CYCLOSPORINE - UNII:83HN0GTJ6D) CYCLOSPORINE 25 mg Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) OLIVE OIL (UNII: 6UYK2W1W1E) FERRIC OXIDE RED (UNII: 1K09F3G675) SORBITOL (UNII: 506T60A25R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) CORN OIL (UNII: 8470G57WFM) CASTOR OIL (UNII: D5340Y2I9G) Product Characteristics Color PINK (pink) Score no score Shape OVAL (Oblong) Size 11mm Flavor Imprint Code 78;240 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0078-0240-15 30 in 1 PACKAGE 03/02/1990 1 NDC:0078-0240-61 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA050625 03/02/1990 SANDIMMUNE
cyclosporine capsule, liquid filledProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0078-0241 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CYCLOSPORINE (UNII: 83HN0GTJ6D) (CYCLOSPORINE - UNII:83HN0GTJ6D) CYCLOSPORINE 100 mg Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) CORN OIL (UNII: 8470G57WFM) FERRIC OXIDE RED (UNII: 1K09F3G675) SORBITOL (UNII: 506T60A25R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) CASTOR OIL (UNII: D5340Y2I9G) OLIVE OIL (UNII: 6UYK2W1W1E) Product Characteristics Color RED (dusty rose) Score no score Shape CAPSULE (Oblong) Size 25mm Flavor Imprint Code 78;241 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0078-0241-15 30 in 1 PACKAGE 03/02/1990 1 NDC:0078-0241-61 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA050625 03/02/1990 SANDIMMUNE
cyclosporine injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0078-0109 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CYCLOSPORINE (UNII: 83HN0GTJ6D) (CYCLOSPORINE - UNII:83HN0GTJ6D) CYCLOSPORINE 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) NITROGEN (UNII: N762921K75) CASTOR OIL (UNII: D5340Y2I9G) 650 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0078-0109-01 10 in 1 BOX 11/14/1983 1 NDC:0078-0109-61 5 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA050573 11/14/1983 SANDIMMUNE
cyclosporine solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0078-0110 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CYCLOSPORINE (UNII: 83HN0GTJ6D) (CYCLOSPORINE - UNII:83HN0GTJ6D) CYCLOSPORINE 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) OLIVE OIL (UNII: 6UYK2W1W1E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0078-0110-22 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 11/14/1983 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA050574 11/14/1983 Labeler - Novartis Pharmaceuticals Corporation (002147023)