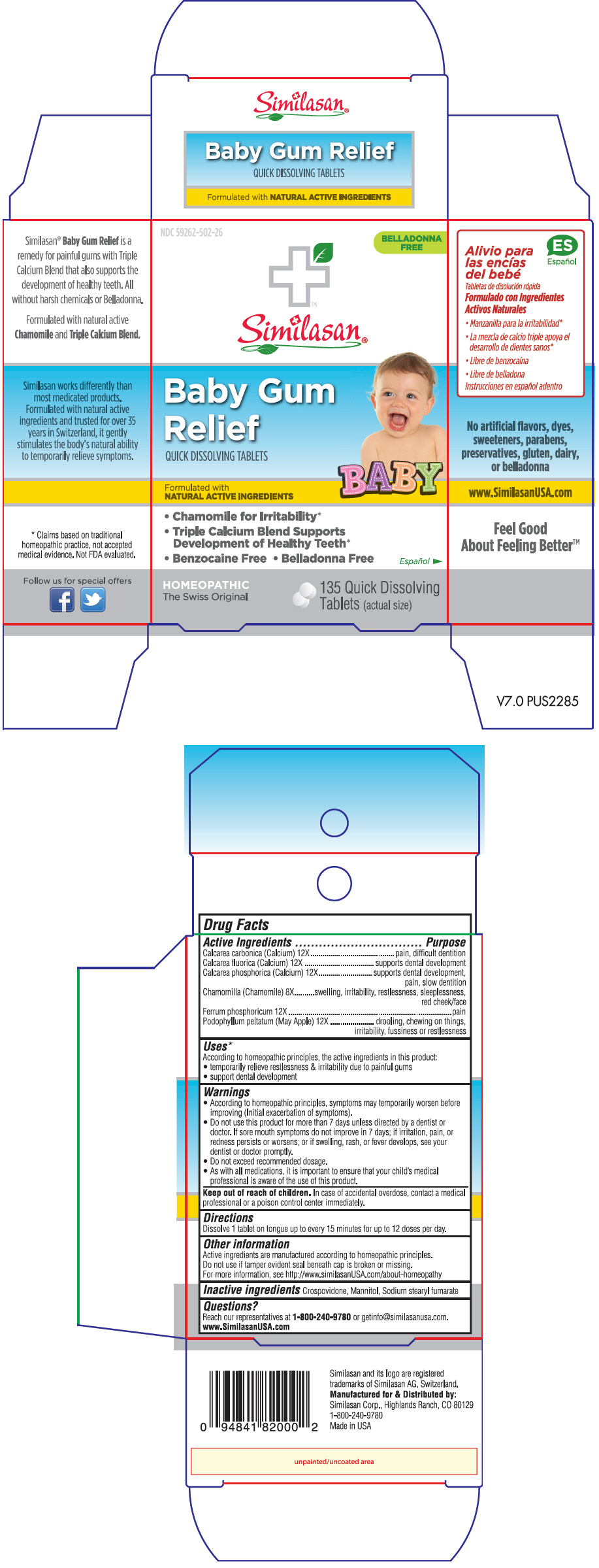
Label: BABY GUM RELIEF- calcium fluoride, chamomile, ferrum phosphoricum, oyster shell calcium carbonate, crude, podophyllum and tribasic calcium phosphate tablet, orally disintegrating
-
Contains inactivated NDC Code(s)
NDC Code(s): 59262-502-26 - Packager: Similasan Corporation
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated August 9, 2018
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
ACTIVE INGREDIENT
Active Ingredients Purpose Calcarea carbonica (Calcium) 12X pain, difficult dentition Calcarea fluorica (Calcium) 12X supports dental development Calcarea phosphorica (Calcium) 12X supports dental development, pain, slow dentition Chamomilla (Chamomile) 8X swelling, irritability, restlessness, sleeplessness, red cheek/face Ferrum phosphoricum 12X pain Podophyllum peltatum (May Apple) 12X drooling, chewing on things, irritability, fussiness or restlessness -
Uses1
According to homeopathic principles, the active ingredients in this product:
- temporarily relieve restlessness & irritability due to painful gums
- support dental development
- 1
- Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
-
Warnings
- According to homeopathic principles, symptoms may temporarily worsen before improving (Initial exacerbation of symptoms).
- Do not use this product for more than 7 days unless directed by a dentist or doctor. If sore mouth symptoms do not improve in 7 days; if irritation, pain, or redness persists or worsens; or if swelling, rash, or fever develops, see your dentist or doctor promptly.
- Do not exceed recommended dosage.
- As with all medications, it is important to ensure that your child's medical professional is aware of the use of this product.
- Directions
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 135 Tablet Bottle Box
NDC 59262-502-26
BELLADONNA
FREESimilasan®
Baby Gum
ReliefQUICK DISSOLVING TABLETS
Formulated with
NATURAL ACTIVE INGREDIENTSBABY
- Chamomile for Irritability*
- Triple Calcium Blend Supports
Development of Healthy Teeth* - Benzocaine Free • Belladonna Free
HOMEOPATHIC
The Swiss Original135 Quick Dissolving
Tablets (actual size)
-
INGREDIENTS AND APPEARANCE
BABY GUM RELIEF
calcium fluoride, chamomile, ferrum phosphoricum, oyster shell calcium carbonate, crude, podophyllum and tribasic calcium phosphate tablet, orally disintegratingProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59262-502 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OYSTER SHELL CALCIUM CARBONATE, CRUDE (UNII: 2E32821G6I) (OYSTER SHELL CALCIUM CARBONATE, CRUDE - UNII:2E32821G6I) OYSTER SHELL CALCIUM CARBONATE, CRUDE 12 [hp_X] CALCIUM FLUORIDE (UNII: O3B55K4YKI) (FLUORIDE ION - UNII:Q80VPU408O) CALCIUM FLUORIDE 12 [hp_X] TRIBASIC CALCIUM PHOSPHATE (UNII: 91D9GV0Z28) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CATION 12 [hp_X] CHAMOMILE (UNII: FGL3685T2X) (CHAMOMILE - UNII:FGL3685T2X) CHAMOMILE 8 [hp_X] FERROSOFERRIC PHOSPHATE (UNII: 91GQH8I5F7) (FERROSOFERRIC PHOSPHATE - UNII:91GQH8I5F7) FERROSOFERRIC PHOSPHATE 12 [hp_X] PODOPHYLLUM (UNII: 2S713A4VP3) (PODOPHYLLUM - UNII:2S713A4VP3) PODOPHYLLUM 12 [hp_X] Inactive Ingredients Ingredient Name Strength CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) MANNITOL (UNII: 3OWL53L36A) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) Product Characteristics Color WHITE Score no score Shape ROUND Size 4mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59262-502-26 1 in 1 BOX 06/15/2018 1 135 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED HOMEOPATHIC 06/15/2018 Labeler - Similasan Corporation (111566530)