Label: CVS OIL FREE ACNE FOAMING SCRUB-PINK GRAPEFRUIT ACNE MEDICATION- salicylic acid lotion
- NDC Code(s): 69842-603-01
- Packager: CVS Health
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 26, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
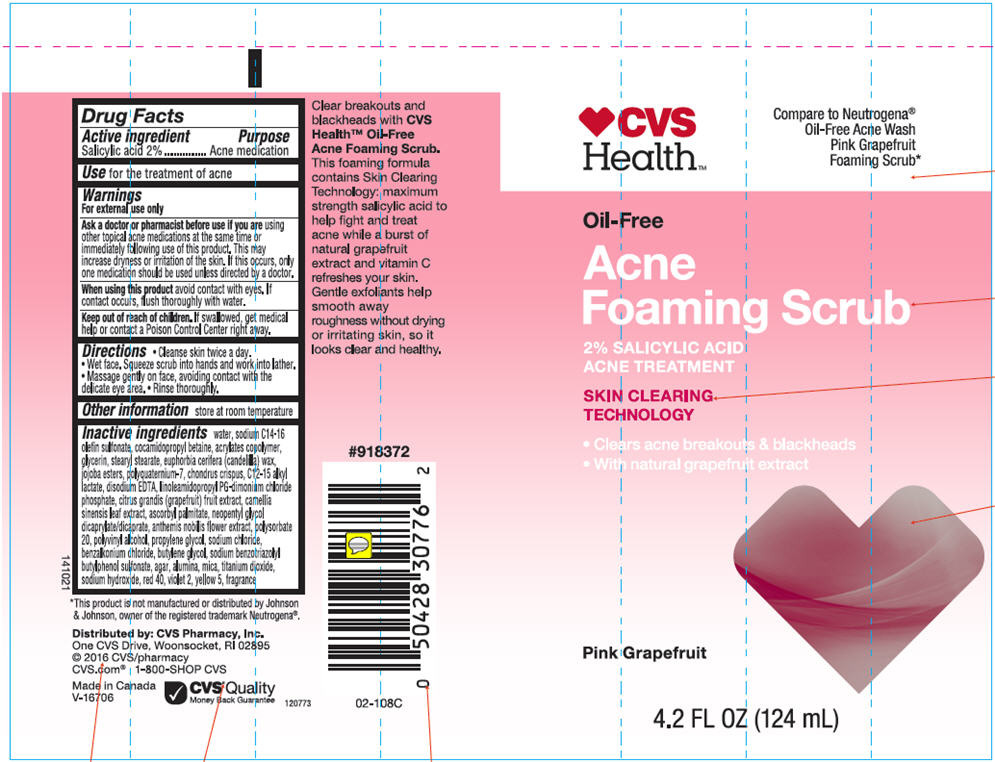
- Drug Facts
- Active ingredients
- Purpose
- Use
-
Warnings
For external use only
- Directions
- Other information
-
Inactive ingredients
Water, Sodium C14-16 Olefin Sulfonate, Cocamidopropyl Betaine, Acrylates Copolymer, Glycerin, Stearyl Stearate, Euphorbia Cerifera (Candelilla) Wax, Jojoba Esters, Polyquaternium-7, Chondrus Crispus, C12-15Alkyl Lactate, Disodium EDTA, Linoleamidopropyl PG-diamonium Chloride Phosphate, Citrus Grandis (Grapefruit) Fruit Extract, Camellia Sinensis Leaf Extract, Ascorbyl Palmitate, Neopentyl Glycol Dicaprilate/Dicaprate, Anthemis Nobilis Flower Extract, Polysorbate 20, Polyvinyl Alcohol, Propylene Glycol, Sodium Chloride, Benzalkonium Chloride, Butylene Glycol, Sodium Benzotriazolyl Butylphenol Sulfonate, Agar, Alumina, Mica, Titanium Dioxide, Sodium Hydroxide, Red 40, Violet 2, Yellow 5, Fragrance.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 124 mL Tube Label
-
INGREDIENTS AND APPEARANCE
CVS OIL FREE ACNE FOAMING SCRUB-PINK GRAPEFRUIT ACNE MEDICATION
salicylic acid lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69842-603 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Salicylic Acid (UNII: O414PZ4LPZ) (Salicylic Acid - UNII:O414PZ4LPZ) Salicylic Acid 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Sodium C14-16 Olefin Sulfonate (UNII: O9W3D3YF5U) Cocamidopropyl Betaine (UNII: 5OCF3O11KX) Polyquaternium-7 (70/30 Acrylamide/dadmac; 1600000 MW) (UNII: 0L414VCS5Y) Glycerin (UNII: PDC6A3C0OX) Stearyl Stearate (UNII: 5WX2EGD0DK) Candelilla Wax (UNII: WL0328HX19) Hydrolyzed Jojoba Esters (Acid Form) (UNII: UDR641JW8W) Chondrus Crispus (UNII: OQS23HUA1X) C12-15 Alkyl Lactate (UNII: GC844VRD7E) Edetate Disodium (UNII: 7FLD91C86K) Linoleamidopropyl Propylene Glycol-Dimonium Chloride Phosphate (UNII: 5Q87K461JO) Pummelo (UNII: ET1TN5W71X) Green Tea Leaf (UNII: W2ZU1RY8B0) Ascorbyl Palmitate (UNII: QN83US2B0N) Neopentyl Glycol Dicaprylate/Dicaprate (UNII: VLW429K27K) Chamaemelum Nobile Flower (UNII: O2T154T6OG) Polysorbate 20 (UNII: 7T1F30V5YH) Polyvinyl Alcohol (100000 MW) (UNII: 949E52Z6MY) Propylene Glycol (UNII: 6DC9Q167V3) Sodium Chloride (UNII: 451W47IQ8X) Benzalkonium Chloride (UNII: F5UM2KM3W7) Butylene Glycol (UNII: 3XUS85K0RA) Sodium Benzotriazolyl Butylphenol Sulfonate (UNII: 0LA2QC9O3Z) Agar, Unspecified (UNII: 89T13OHQ2B) Aluminum Oxide (UNII: LMI26O6933) Mica (UNII: V8A1AW0880) Titanium Dioxide (UNII: 15FIX9V2JP) Sodium Hydroxide (UNII: 55X04QC32I) FD&C Red No. 40 (UNII: WZB9127XOA) D&C Violet No. 2 (UNII: 350KA7O6HK) FD&C Yellow No. 5 (UNII: I753WB2F1M) Product Characteristics Color PINK Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69842-603-01 124 mL in 1 TUBE; Type 0: Not a Combination Product 01/01/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part333D 01/01/2014 Labeler - CVS Health (062312574) Registrant - Garcoa, Inc. (036464697) Establishment Name Address ID/FEI Business Operations Garcoa, Inc. 036464697 MANUFACTURE(69842-603)