Label: ROBITUSSIN 12 HOUR COUGH RELIEF- dextromethorphan polistirex suspension, extended release
- NDC Code(s): 0031-8655-10, 0031-8753-03, 0031-8754-15, 0031-8755-15
- Packager: Haleon US Holdings LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated April 8, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
Do not useif you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- chronic cough that lasts as occurs with smoking, asthma, or emphysema
- cough that occurs with too much phlegm (mucus)
-
DOSAGE & ADMINISTRATION
Directions
- shake bottle well before use
- measure only with dosing cup provided. Do not use dosing cup with other products
- dose as follows or as directed by doctor
- mL = milliliter
adults and children 12 years of age and over
10 mL every 12 hours, not to exceed 20 mL in 24 hours
children 6 to under 12 years of age
5 mL every 12 hours, not to exceed 10 mL in 24 hours
children 4 to under 6 years of age
2.5 mL every 12 hours, not to exceed 5 mL in 24 hours
children under 4 years of age
do not use
- STORAGE AND HANDLING
-
INACTIVE INGREDIENT
Inactive ingredients(Grape flavor)
D&C red no. 30, FD&C blue no. 1, flavor, glycerin, high fructose corn syrup, methylparaben, polysorbate 80, polyvinyl acetate, povidone, propylparaben, purified water, sodium metabisulfite, sodium polystyrene sulfonate, sucrose, tartaric acid, tragacanth gum, triacetin, xanthan gum
-
INACTIVE INGREDIENT
Inactive ingredients(Orange flavor)
D&C red no. 30, D&C yellow no. 10, flavor, glycerin, high fructose corn syrup, methylparaben, polysorbate 80, polyvinyl acetate, povidone, propylparaben, purified water, sodium metabisulfite, sodium polystyrene sulfonate, sucrose, tartaric acid, tragacanth gum, triacetin, xanthan gum
- QUESTIONS
- SPL UNCLASSIFIED SECTION
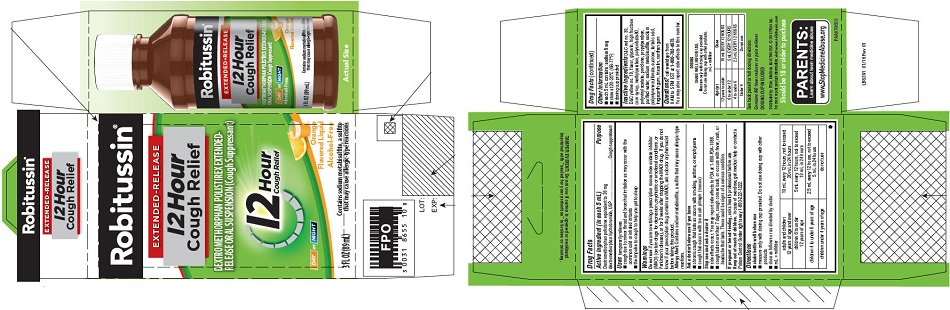
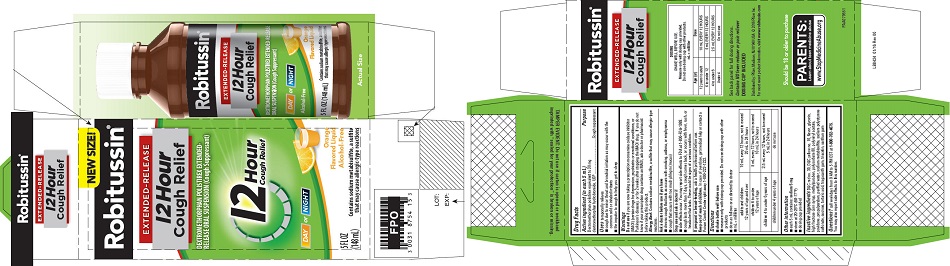
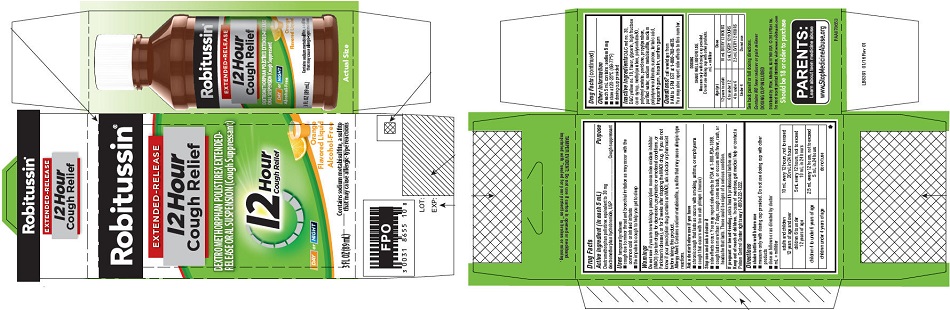
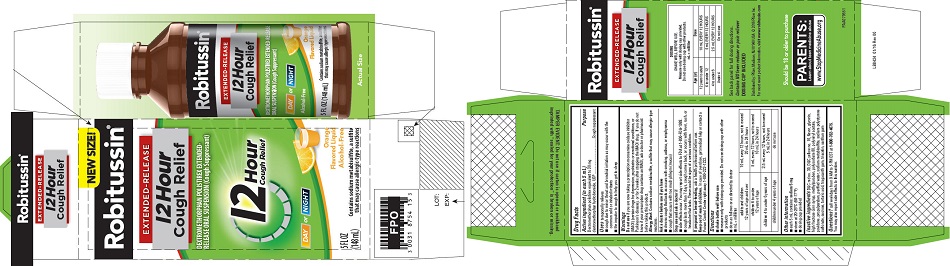
- PRINCIPAL DISPLAY PANEL
-
PRINCIPAL DISPLAY PANEL
NEW SIZE!
Robitussin ®
EXTENDED-RELEASE
12 Hour
Cough ReliefDEXTROMETHORPHAN POLISTIREX EXTENDED-
RELEASE ORAL SUSPENSION (Cough Suppressant)12 Hour
Cough ReliefDAY or NIGHT
Orange
Flavored LiquidAlcohol-Free
5 FL OZ
(148 mL)Contains sodium metabisulfite, a sulfite
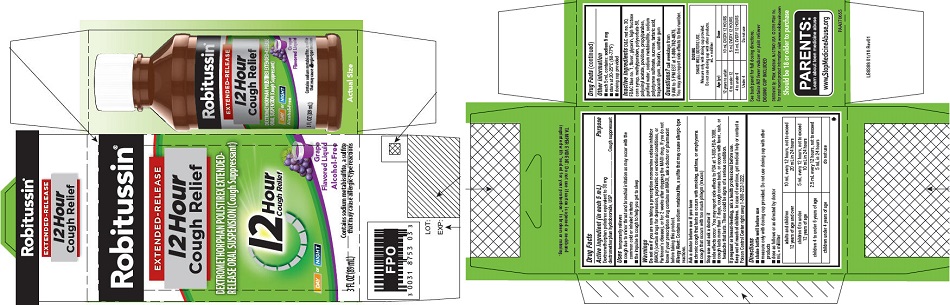
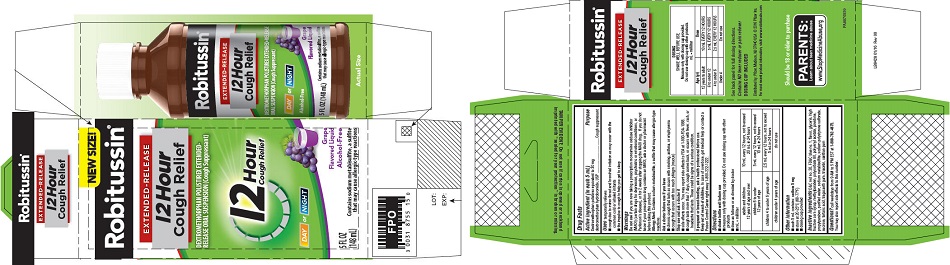
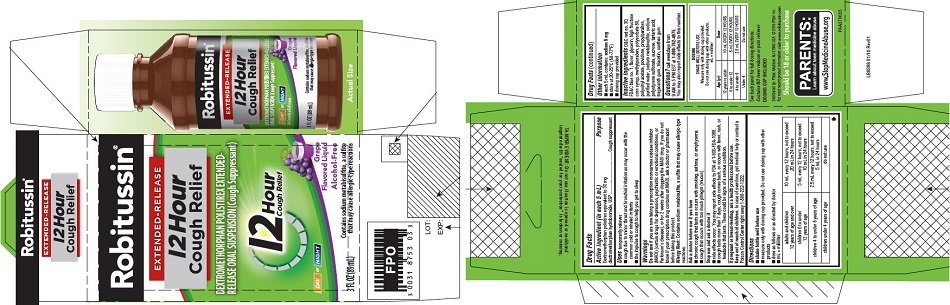
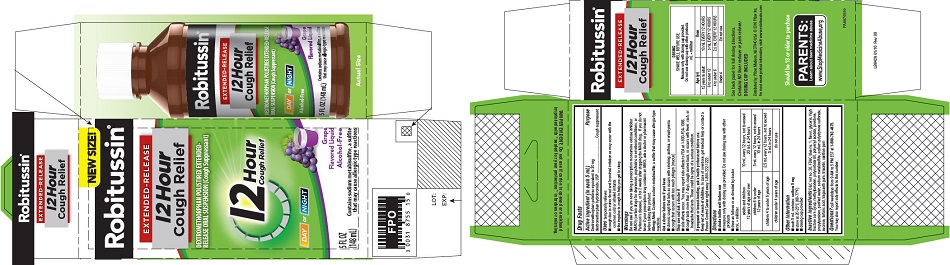
that may cause allergic-type reactions - PRINCIPAL DISPLAY PANEL
-
PRINCIPAL DISPLAY PANEL
NEW SIZE!
Robitussin ®
EXTENDED-RELEASE
12 Hour
Cough ReliefDEXTROMETHORPHAN POLISTIREX EXTENDED-
RELEASE ORAL SUSPENSION (Cough Suppressant)12 Hour
Cough ReliefDAY or NIGHT
Grape
Flavored LiquidAlcohol-Free
5 FL OZ
(148 mL)Contains sodium metabisulfite, a sulfite
that may cause allergic-type reactions -
INGREDIENTS AND APPEARANCE
ROBITUSSIN 12 HOUR COUGH RELIEF
dextromethorphan polistirex suspension, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0031-8655 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 30 mg in 5 mL Inactive Ingredients Ingredient Name Strength POLISTIREX (UNII: 5H9W9GTW27) D&C RED NO. 30 (UNII: 2S42T2808B) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) GLYCERIN (UNII: PDC6A3C0OX) HIGH FRUCTOSE CORN SYRUP (UNII: XY6UN3QB6S) METHYLPARABEN (UNII: A2I8C7HI9T) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POLYVINYL ACETATE (UNII: 32K497ZK2U) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM METABISULFITE (UNII: 4VON5FNS3C) SODIUM POLYSTYRENE SULFONATE (UNII: 1699G8679Z) SUCROSE (UNII: C151H8M554) TARTARIC ACID (UNII: W4888I119H) TRAGACANTH (UNII: 2944357O2O) TRIACETIN (UNII: XHX3C3X673) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color orange Score Shape Size Flavor ORANGE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0031-8655-10 1 in 1 CARTON 07/01/2015 1 89 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091135 07/01/2015 ROBITUSSIN 12 HOUR COUGH RELIEF
dextromethorphan polistirex suspension, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0031-8754 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 30 mg in 5 mL Inactive Ingredients Ingredient Name Strength POLISTIREX (UNII: 5H9W9GTW27) D&C RED NO. 30 (UNII: 2S42T2808B) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) GLYCERIN (UNII: PDC6A3C0OX) HIGH FRUCTOSE CORN SYRUP (UNII: XY6UN3QB6S) METHYLPARABEN (UNII: A2I8C7HI9T) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POLYVINYL ACETATE (UNII: 32K497ZK2U) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM METABISULFITE (UNII: 4VON5FNS3C) SODIUM POLYSTYRENE SULFONATE (UNII: 1699G8679Z) SUCROSE (UNII: C151H8M554) TARTARIC ACID (UNII: W4888I119H) TRAGACANTH (UNII: 2944357O2O) TRIACETIN (UNII: XHX3C3X673) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color orange Score Shape Size Flavor ORANGE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0031-8754-15 1 in 1 CARTON 07/05/2016 1 148 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091135 07/05/2016 ROBITUSSIN 12 HOUR COUGH RELIEF
dextromethorphan polistirex suspension, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0031-8753 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 30 mg in 5 mL Inactive Ingredients Ingredient Name Strength POLISTIREX (UNII: 5H9W9GTW27) D&C RED NO. 30 (UNII: 2S42T2808B) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GLYCERIN (UNII: PDC6A3C0OX) HIGH FRUCTOSE CORN SYRUP (UNII: XY6UN3QB6S) METHYLPARABEN (UNII: A2I8C7HI9T) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POLYVINYL ACETATE (UNII: 32K497ZK2U) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM METABISULFITE (UNII: 4VON5FNS3C) SODIUM POLYSTYRENE SULFONATE (UNII: 1699G8679Z) SUCROSE (UNII: C151H8M554) TARTARIC ACID (UNII: W4888I119H) TRAGACANTH (UNII: 2944357O2O) TRIACETIN (UNII: XHX3C3X673) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color purple Score Shape Size Flavor GRAPE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0031-8753-03 1 in 1 CARTON 07/01/2015 1 89 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091135 07/01/2015 ROBITUSSIN 12 HOUR COUGH RELIEF
dextromethorphan polistirex suspension, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0031-8755 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 30 mg in 5 mL Inactive Ingredients Ingredient Name Strength POLISTIREX (UNII: 5H9W9GTW27) D&C RED NO. 30 (UNII: 2S42T2808B) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GLYCERIN (UNII: PDC6A3C0OX) HIGH FRUCTOSE CORN SYRUP (UNII: XY6UN3QB6S) METHYLPARABEN (UNII: A2I8C7HI9T) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POLYVINYL ACETATE (UNII: 32K497ZK2U) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM METABISULFITE (UNII: 4VON5FNS3C) SODIUM POLYSTYRENE SULFONATE (UNII: 1699G8679Z) SUCROSE (UNII: C151H8M554) TARTARIC ACID (UNII: W4888I119H) TRAGACANTH (UNII: 2944357O2O) TRIACETIN (UNII: XHX3C3X673) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color purple Score Shape Size Flavor GRAPE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0031-8755-15 1 in 1 CARTON 07/05/2016 1 148 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091135 07/05/2016 Labeler - Haleon US Holdings LLC (079944263)