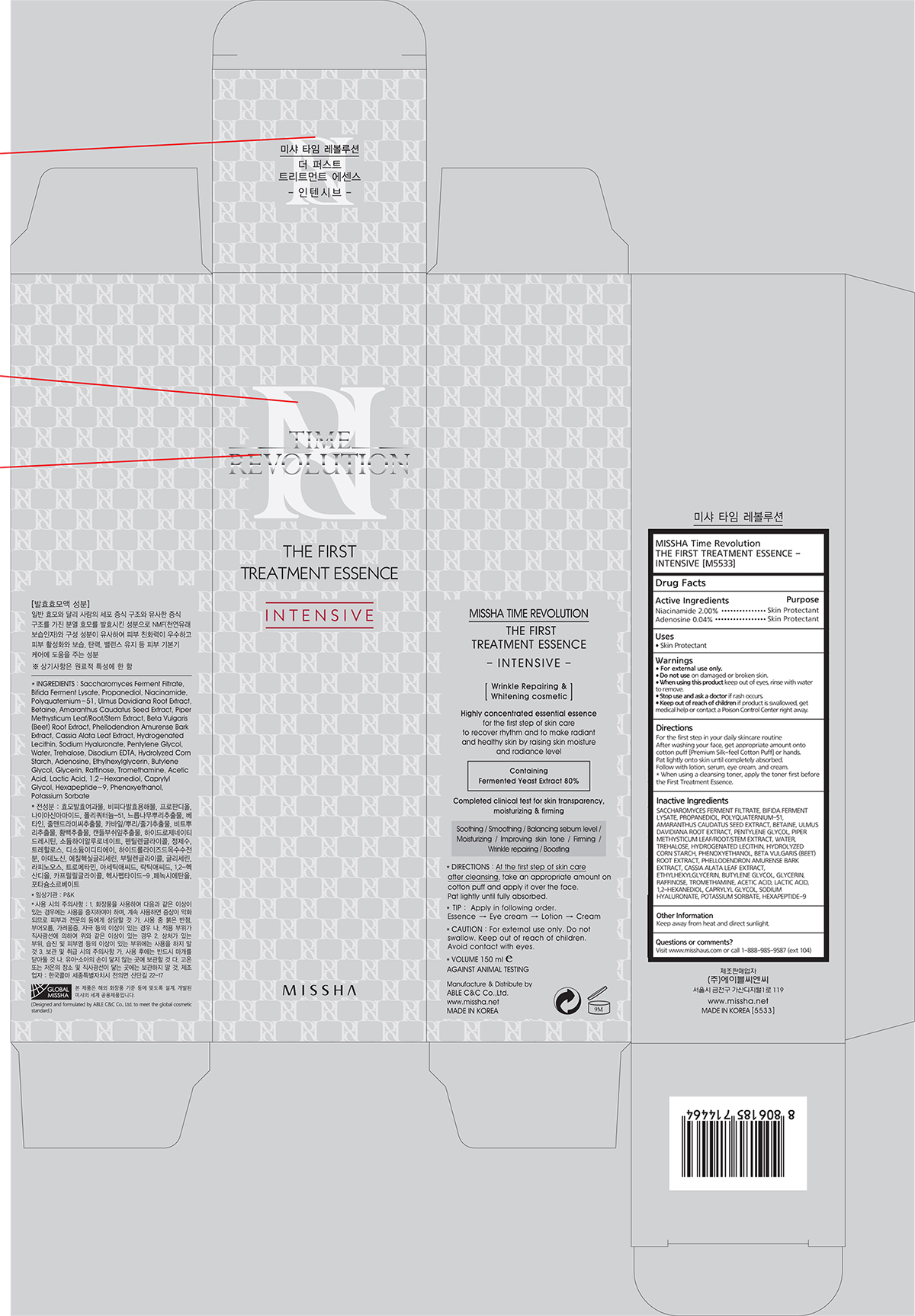
MISSHA TIME REVOLUTION THE FIRST TREATMENT ESSENCE INTENSIVE- niacinamide, adenosine liquid
Able C&C Co., Ltd.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Active Ingredients
Niacinamide 2.00%
Adenosine 0.04%
Directions
For the first step in your daily skincare routine
After washing your face, get appropriate amount onto cotton puff [Premium Silk-feel Cotton Puff] or hands.
Pat lightly onto skin until completely absorbed.
Follow with lotion, serum, eye cream, and cream.
Warnings
For external use only.
Do not use
Do not use on damaged or broken skin.
When using this product
When using this product keep out of eyes, rinse with water to remove.
Stop use
Stop use and ask a doctor if rash occurs.
Keep out of reach of children
Keep out of reach of children if product is swallowed, get medical help or contact a Poison Control Center right away.
Inactive Ingredients
SACCHAROMYCES FERMENT FILTRATE, IFIDA FERMENT LYSATE, PROPANEDIOL, POLYQUATERNIUM-51, AMARANTHUS CAUDATUS SEED EXTRACT, BETAINE, ULMUS DAVIDIANA ROOT EXTRACT, PENTYLENE GLYCOL, PIPER METHYSTICUM LEAF/ROOT/STEM EXTRACT, WATER, TREHALOSE, HYDROGENATED LECITHIN, HYDROLYZED CORN STARCH, PHENOXYETHANOL, BETA VULGARIS (BEET) ROOT EXTRACT, PHELLODENDRON AMURENSE BARK EXTRACT, CASSIA ALATA LEAF EXTRACT, ETHYLHEXYLGLYCERIN, BUTYLENE GLYCOL, GLYCERIN, RAFFINOSE, TROMETHAMINE, ACETIC ACID, LACTIC ACID, 1,2-HEXANEDIOL, CAPRYLYL GLYCOL, SODIUM HYALURONATE, POTASSIUM SORBATE, HEXAPEPTIDE-9
Other Information
Keep away from heat and direct sunlight.
Questions or comments?
Visit www.misshaus.com or call 1-888-985-9587 (ext 104)
MISSHA Time Revolution The First Treatment Essence Intensive
Able C&C Co., Ltd.
