SULFO LO- sulfur soap
Bradford Soap Works, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
sulfo lo ® Cleansing Bar
Indications & Useage
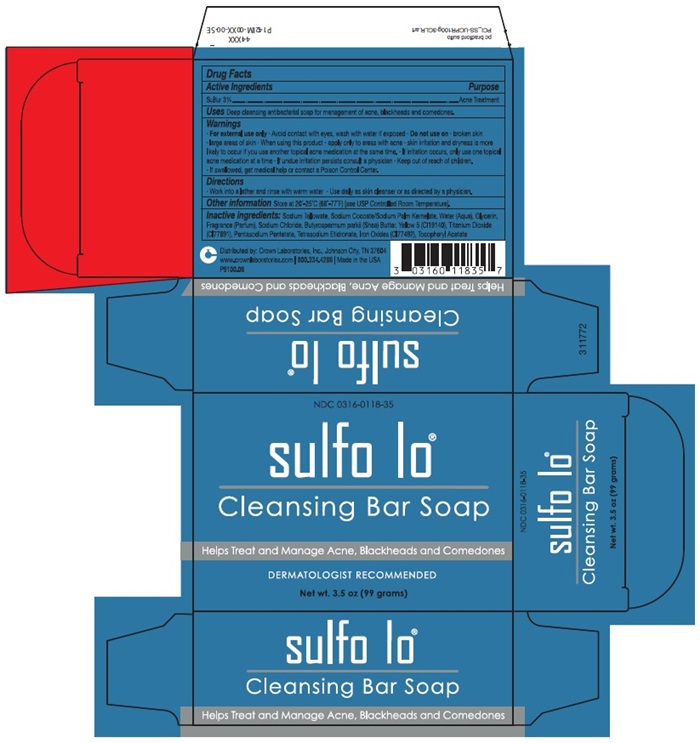
Deep cleansing antibacterial soap for management of acne, blackheads and comedones.
Warnings
- For external use only • Avoid contact with eyes, was with water if exposed • Do not use on • broken skin • large areas of skin • skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time • If irritation occurs, only use one topical acne medication at a time • If undue irritation persists consult a physician • Keep out of reach of children. • if swallowed, get medical help or contact a Poison Control Center.
Inactive Ingredient
Sodium Tallowate, Sodium Cocoate/Sodium Palm Kernelate, Water (Aqua), Glycerin, Fragrance (Parfum), Sodium Chloride, Butyrospermum parkii (Shea) Butter, Yellow 5 (CI19140), Titanium Dioxide (CI77891), Pentasodium Pentetate, Tetrasodium Etidonate, Iron Oxides (CI77492), Tocopheryl Acetate
Dosage & Administration
- Work into a lather and rinse with warm water • Use daily as skin cleanser or as directed by a physician.
|
Drug Facts Active Ingredients Purpose | |
|
Sulfur 3% ……………………………………………………………………………………………… Acne Treatment Uses Deep cleansing antibacterial soap for management of acne, blackheads and comedones. Warnings
Directions
Other Information Store at 20°-25°C (68°-77°F) (see USP Controlled Room Temperature Inactive Ingredients: Sodium Tallowate, Sodium Cocoate/Sodium Palm Kernelate, Water (Aqua), Glycerin, Fragrance (Parfum), Sodium Chloride, Butyrospermum parkii (Shea) Butter, Yellow 5 (CI19140), Titanium Dioxide (CI77891), Pentasodium Pentetate, Tetrasodium Etidonate, Iron Oxides (CI77492), Tocopheryl Acetate Distributed by: Crown Laboratories, Inc., Johnson City, TN 37604 www.crownlaboratories.com | 800.334.4286 | Made in the USA P9100.06 | |
|
sulfo lo® Cleansing Bar Soap Helps Treat and Manage Acne, Blackheads and Comedones | |
|
NDC 0316-0118-35 sulfo lo® Cleansing Bar Soap Helps Treat and Manage Acne, Blackheads and Comedones DERMATOLOGIST RECOMMENDED Net wt. 3.5 oz (99 grams) |
NDC 0316-0118-35 sulfo lo® Cleansing Bar Soap Net wt. 3.5 oz (99 grams) |
|
sulfo lo® Cleansing Bar Soap Helps Treat and Manage Acne, Blackheads and Comedones |
| SULFO LO
sulfur soap |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Bradford Soap Works, Inc. (001201045) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bradford Soap Works, Inc. | 001201045 | manufacture(11118-0007) | |