Label: COLD AND FLU DAYTIME SEVERE- acetaminophen, dextromethorphan hbr, guaifenesin, phenylephrine hcl tablet, film coated
- NDC Code(s): 41250-643-08
- Packager: Meijer Distribution Inc
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated January 15, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
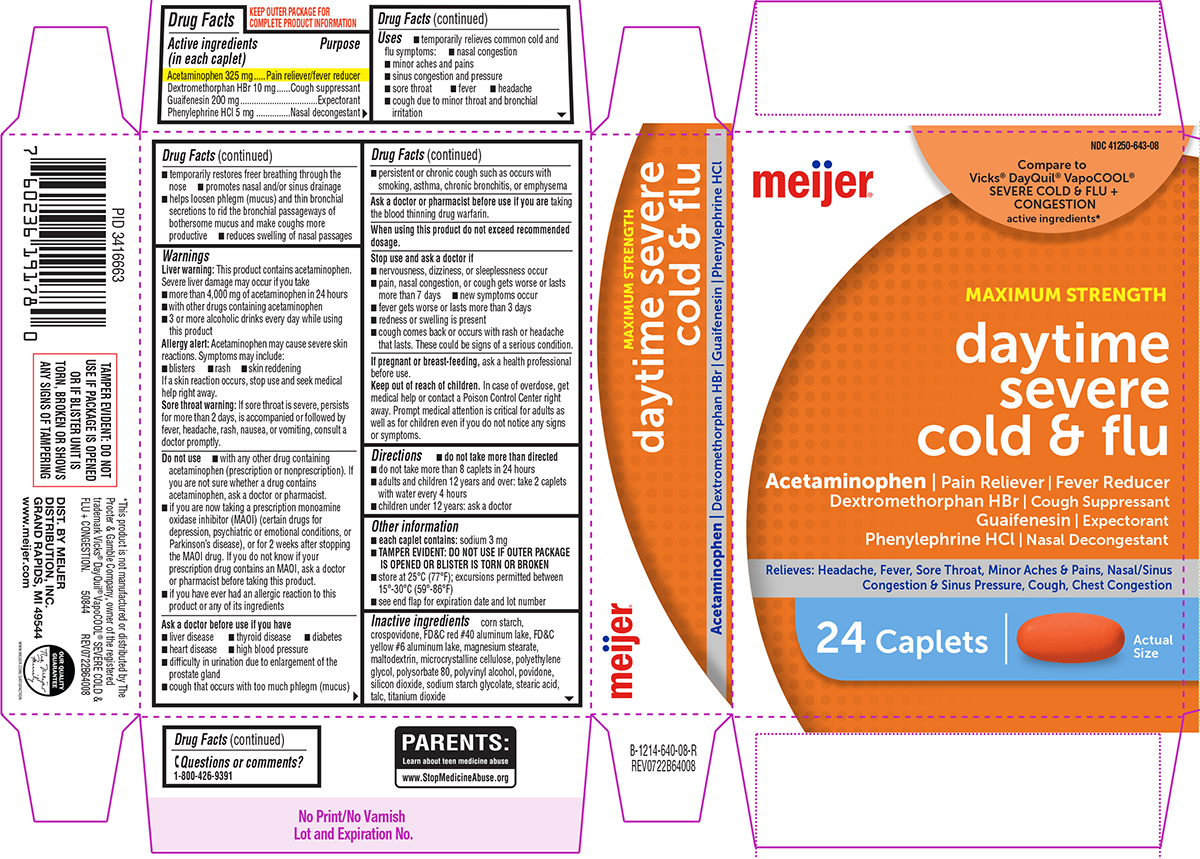
- Active ingredients (in each caplet)
- Purpose
-
Uses
- temporarily relieves common cold and flu symptoms:
- minor aches and pains
- sinus congestion and pressure
- sore throat
- fever
- headache
- nasal congestion
- cough due to minor throat and bronchial irritation
- reduces swelling of nasal passages
- temporarily restores freer breathing through the nose
- promotes nasal and/or sinus drainage
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
- temporarily relieves common cold and flu symptoms:
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- blisters
- rash
- skin reddening
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if you have
- liver disease
- thyroid disease
- diabetes
- heart disease
- high blood pressure
- difficulty in urination due to enlargement of the prostate gland
- cough that occurs with too much phlegm (mucus)
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
Stop use and ask a doctor if
- nervousness, dizziness, or sleeplessness occur
- pain, nasal congestion, or cough gets worse or lasts more than 7 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present
- new symptoms occur
- cough comes back or occurs with rash or headache that lasts. These could be signs of a serious condition.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
meijer®
NDC 41250-643-08
Compare to
Vicks® DayQuil® VapoCOOL®
SEVERE COLD & FLU +
CONGESTION
active ingredients*
MAXIMUM STRENGTH
daytime
severe
cold & flu
Acetaminophen | Pain Reliever | Fever Reducer
Dextromethorphan HBr | Cough Suppressant
Guaifenesin | Expectorant
Phenylephrine HCl | Nasal DecongestantRelieves: Headache, Fever, Sore Throat, Minor Aches & Pains,
Nasal/Sinus Congestion & Sinus Pressure, Cough, Chest Congestion24 Caplets
Actual
SizeTAMPER EVIDENT: DO NOT
USE IF PACKAGE IS OPENED
OR IF BLISTER UNIT IS
TORN, BROKEN OR SHOWS
ANY SIGNS OF TAMPERING*This product is not manufactured or distributed by The
Procter & Gamble Company, owner of the registered
trademark Vicks® DayQuil® VapoCOOL® SEVERE COLD &
FLU + CONGESTION. 50844 REV0722B64008DIST. BY MEIJER
DISTRIBUTION, INC.
GRAND RAPIDS, MI 49544
www.meijer.comPARENTS:
Learn about teen medicine abuse
www.StopMedicineAbuse.orgOUR QUALITY
GUARANTEE
The Meijer
Family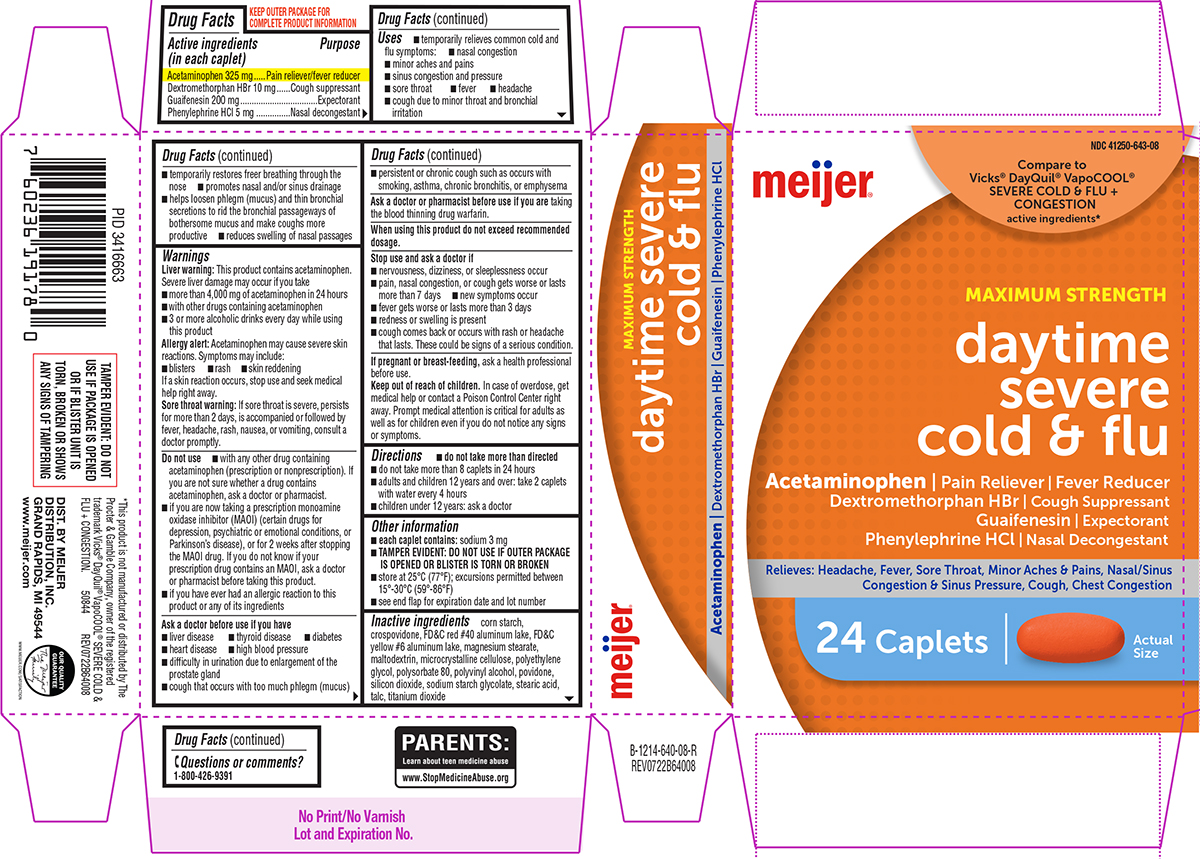
meijer 44-640
-
INGREDIENTS AND APPEARANCE
COLD AND FLU DAYTIME SEVERE
acetaminophen, dextromethorphan hbr, guaifenesin, phenylephrine hcl tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41250-643 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 10 mg GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 200 mg PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 5 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) STEARIC ACID (UNII: 4ELV7Z65AP) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color orange Score no score Shape OVAL Size 19mm Flavor Imprint Code 44;640 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41250-643-08 2 in 1 CARTON 05/08/2020 1 12 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 05/08/2020 Labeler - Meijer Distribution Inc (006959555) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 manufacture(41250-643) , pack(41250-643) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867894 manufacture(41250-643) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 117025878 manufacture(41250-643)