EYE DROPS LUBRICATING TEARS- hypromellose, glycerin, polyethylene glycol 400 solution/ drops
KC Pharmaceuticals, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Keep out of reach of children.
If accidentally swallowed, get medical help or contact a Poison Control Center immediately.
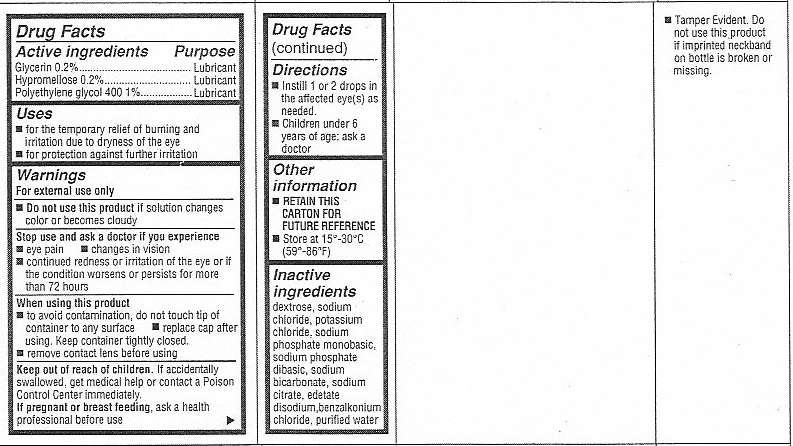
for the temporary relief of burning and irritation due to dryness of the eye
for protection against further irritation
Warnings
For external use only
Do not use this product if solution changes color or becomes cloudy
Directions
Instill 1 or 2 drops in the affected eye(s) as needed.
Children under 6 years of age: ask a doctor
Inactive Ingredients
dextrose, sodium chloride, potassium chloride, sodium phosphate monobasic, sodium phosphate dibasic, sodium bicarbonate, sodium citrate, edetate disodium, benzalkonium chloride, purified water
| EYE DROPS LUBRICATING TEARS
hypromellose, glycerin, polyethylene glycol 400 solution/ drops |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - KC Pharmaceuticals, Inc. (174450460) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| KC Pharmaceuticals, Inc. | 174450460 | manufacture(55651-026) , pack(55651-026) , label(55651-026) | |