CEBATROL OIL CONTROL PADS- salicylic acid solution
ZO MEDICAL CEBATROL OIL CONTROL PADS ACNE TREATMENT- salicylic acid solution
ZO Skin Health, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
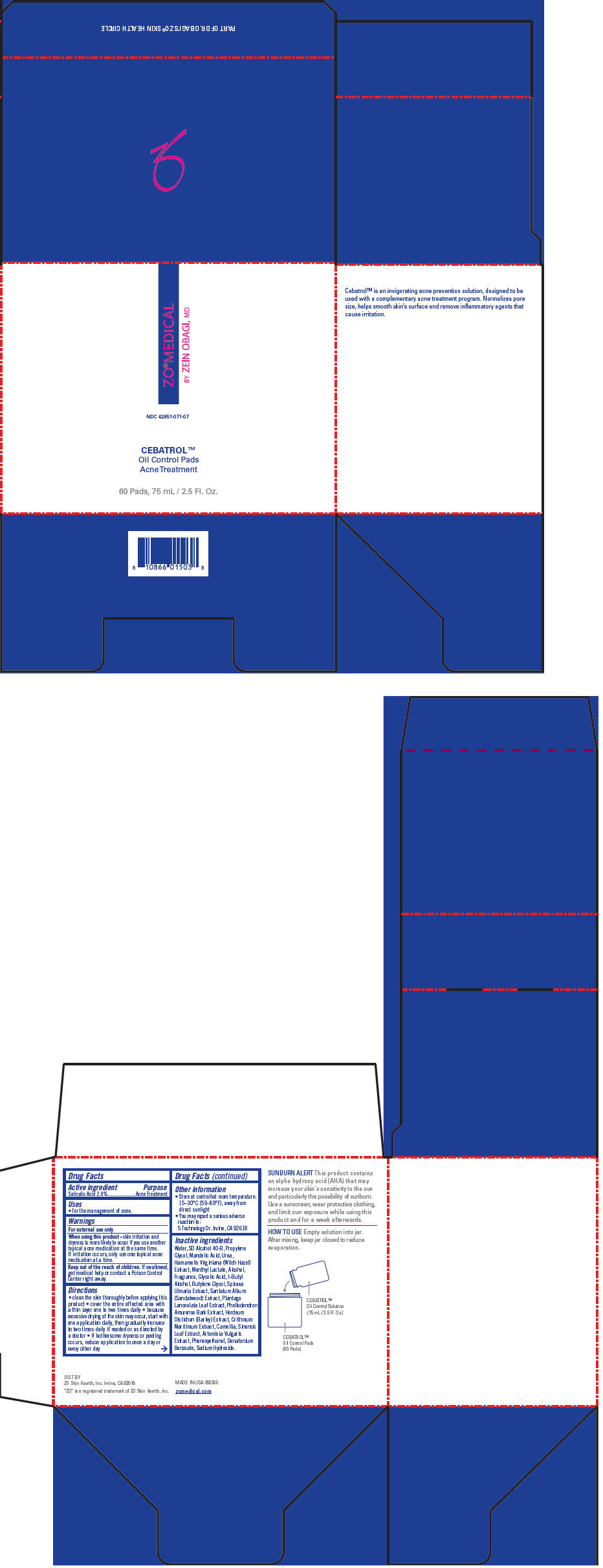
Active ingredient
Salicylic Acid 2.0%
Uses
- for the management of acne.
Warnings
For external use only
When using this product
- skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
Keep out of the reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- clean the skin thoroughly before applying this product
- cover the entire affected area with a thin layer one to two times daily
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
Other information
- Store at controlled room temperature: 15–30°C (59–89°F), away from direct sunlight
- You may report a serious adverse reaction to:
5 Technology Dr. Irvine, CA 92618
Inactive ingredients
Water, SD Alcohol 40-B, Propylene Glycol, Mandelic Acid, Urea, Hamamelis Virginiana (Witch Hazel) Extract, Menthyl Lactate, Alcohol, Fragrance, Glycolic Acid, t-Butyl Alcohol, Butylene Glycol, Spiraea Ulmaria Extract, Santalum Album (Sandalwood) Extract, Plantago Lanceolata Leaf Extract, Phellodendron Amurense Bark Extract, Hordeum Distichon (Barley) Extract, Crithmum Maritimum Extract, Camellia, Sinensis Leaf Extract, Artemisia Vulgaris Extract, Phenoxyethanol, Denatonium Benzoate, Sodium Hydroxide.
DIST BY
ZO Skin Health, Inc. Irvine, CA 92618
PRINCIPAL DISPLAY PANEL - 75 mL Bottle Carton
ZO®MEDICAL
BY ZEIN OBAGI, MD
NDC 42851-071-07
CEBATROL™
Oil Control Pads
Acne Treatment
60 Pads, 75 mL / 2.5 Fl. Oz.
PRINCIPAL DISPLAY PANEL - 20 Packet Carton
ZO®MEDICAL
BY ZEIN OBAGI, MD
NDC 42851-075-00
CEBATROL™
Oil Control Pads
Acne Treatment
20 Single-Use Packettes (1mL / 0.03 Fl. Oz. each)
ZO Skin Health, Inc.