CAPSAICIN- capsaicin cream
7T Pharma LLC
----------
Capsaicin Cream
CAPSAICIN – Capsaicin 0.025% Cream
7T Pharma, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
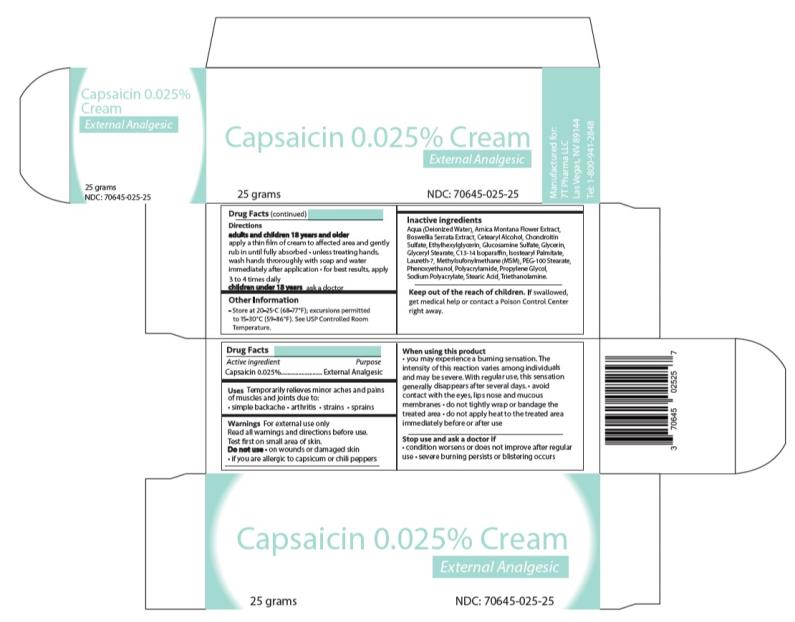
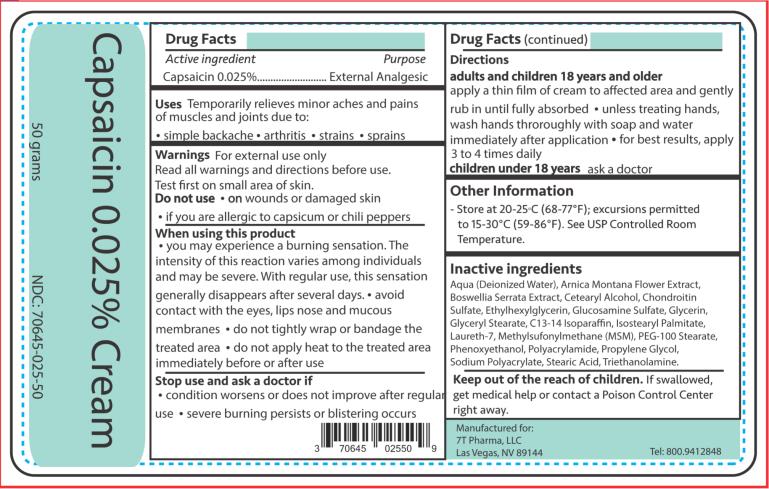
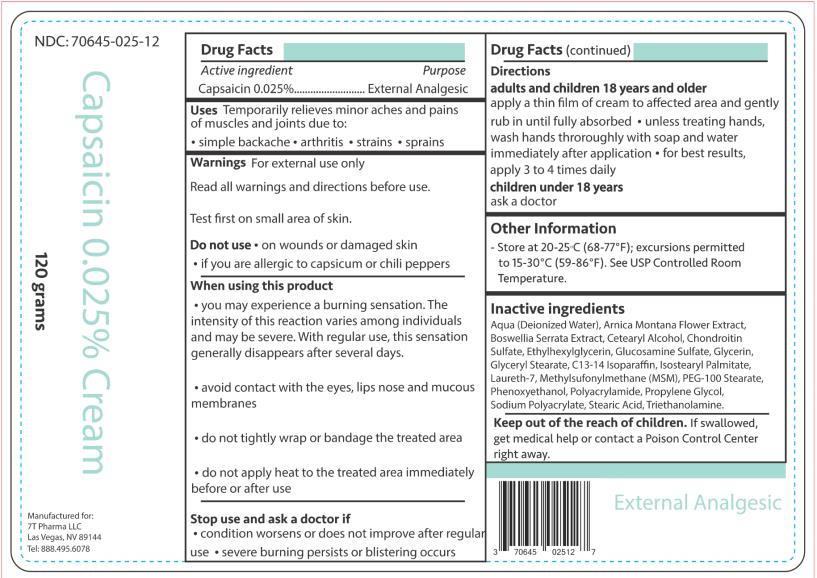
Capsaicin 0.025% Cream
Drug Facts
Uses
Temporarily relieves minor aches and pains of muscles and joints due to:
• simple backache
• arthritis
• strains
• sprains
Warnings
For external use only
Read all warnings and directions before use. Test first on small area of skin.
When using this product
• You may experience a burning sensation. The intensity of this reaction varies among individuals and may be severe. With regular use, this sensation generally disappears after several days.
• Avoid contact with the eyes, lips, nose and mucous membranes
• Do not tightly wrap or bandage the treated area
• Do not apply heat to the treated area immediately before or after use
Directions
Adults and children 18 years of age and older:
Apply a thin film of cream to affected area and gently rub in until fully absorbed unless treating hands. Wash hands thoroughly with soap and water immediately after application for best results. Apply 3 to 4 times daily.
Children under 18 years: Ask a doctor
Inactive ingredients
Aqua (Deionized Water), Arnica Montana Flower Extract, Boswellia Serrata Extract, Cetearyl Alcohol, Chondroitin Sulfate, Ethylhexylglycerin, Glucosamine Sulfate, Glycerin, Glyceryl Stearate, C13-14 Isoparaffin, Isostearyl Palmitate, Laureth-7, Methylsufonylmethane (MSM), PEG-100 Stearate, Phenoxyethanol, Polyacrylamide, Propylene Glycol, Sodium Polyacrylate, Stearic Acid, and Triethanolamine
| CAPSAICIN
capsaicin cream |
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
| Labeler - 7T Pharma LLC (080220022) |