SODIUM CITRATE AND CITRIC ACID- sodium citrate and citric acid monohydrate solution
Westminster Pharmaceuticals, LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Sodium Citrate and Citric Acid
Oral Solution USP
DESCRIPTION
Sodium Citrate and Citric Acid Oral Solution USP is a stable and pleasant-tasting systemic alkalizer containing sodium citrate and citric acid in a sugar-free base.
It is a nonparticulate neutralizing buffer.
Sodium Citrate and Citric Acid Oral Solution USP contains in each teaspoonful (5 mL):
| Sodium Citrate Dihydrate | 500 mg |
| Citric Acid Monohydrate | 334 mg |
Each mL contains 1 mEq Sodium Ion, and is equivalent to 1 mEq Bicarbonate (HCO3 ).
CLINICAL PHARMACOLOGY
Sodium citrate is absorbed and metabolized to sodium bicarbonate, thus acting as a systemic alkalizer. The effects are essentially those of chlorides before absorption and those of bicarbonates subsequently. Oxidation is virtually complete so that less than 5% of sodium citrate is excreted in the urine unchanged.
INDICATIONS AND USAGE
Sodium Citrate and Citric Acid Oral Solution USP is an effective alkalinizing agent. It is useful in those conditions where long-term maintenance of an alkaline urine is desirable, and is of value in the alleviation of chronic metabolic acidosis, such as results from chronic renal insufficiency or the syndrome of renal tubular acidosis, especially when the administration of potassium salts is undesirable or contraindicated. This product is also useful for buffering and neutralizing gastric hydrochloric acid quickly and effectively.
Sodium Citrate and Citric Acid Oral Solution USP is concentrated, and when administered after meals and before bedtime, allows one to maintain an alkaline urinary pH around the clock, usually without the necessity of a 2 A.M. dose. This product alkalinizes the urine without producing a systemic alkalosis in the recommended dosage.This product is highly palatable, pleasant tasting,and tolerable, even when a administered for long periods.
CONTRAINDICATIONS
Contraindicated in patients with sodium-restricted diets, with severe renal impairment, or known hypersensitivity to any of the ingredients.
PRECAUTIONS
Sodium Citrate and Citrict Acid Oral Solution USP should be used with caution by patients with low urinary output unless under the supervision of a physician. This product should not be administered concurrently with aluminum-based antacids. Patients should be directed to dilute adequately with water and preferably, to take each dose after meals to avoid saline laxative effect. Sodium salts should be used cautiously in patients with cardiac failure, hypertension, impaired renal function, peripheral and pulmonary edema, and toxemia of pregnancy.
Periodic examinations and determinations of serum electrolytes, particularly serum bicarbonate level, should be carried out in those patients with renal disease in order to avoid these complications
ADVERSE REACTIONS
Sodium Citrate and Citric Acid Oral Solution USP is generally well tolerated, without any unpleasant side effects, when given in recommended doses to patients with normal renal function and urinary output. However, as with any alkalinizing agent, caution must be used in certain patients with abnormal renal mechanisms to avoid development of alkalosis, especially in the presence of hypocalcemia.
OVERDOSAGE
Overdosage with sodium salts may cause diarrhea, nausea, and vomiting, hypernoia, and convulsions.
DOSAGE AND ADMINISTRATION
Sodium Citrate and Citric Acid Oral Solution USP should be administered by diluting in water, followed by additional water, if desired.
SHAKE WELL BEFORE USING.
For Systemic Alkalization:
Usual Adult Dosage: 2 to 6 teaspoonfuls (10 to 30 mL), diluted in 1 to 3 ounces of water, after meals and at bedtime, or as directed by a physician.
Usual Pediatric Dose: 1 to 3 teaspoonfuls (5 to 15 mL), diluted in 1 to 3 ounces of water,after meals and at bedtime, or as directed by a physician.
For children under two years of age, use is based on consultation with a physician.
As a neutralizing buffer: 3 teaspoonfuls (15 mL), diluted with 15 mL water, taken as a single dose, or as directed by a physician.
HOW SUPPLIED
Sodium Citrate and Citric Acid Oral Solution USP is a (colorless, grape flavor) solution and is supplied in the following oral dosage form:
NDC 69367-320-16 (16 fl oz bottles).
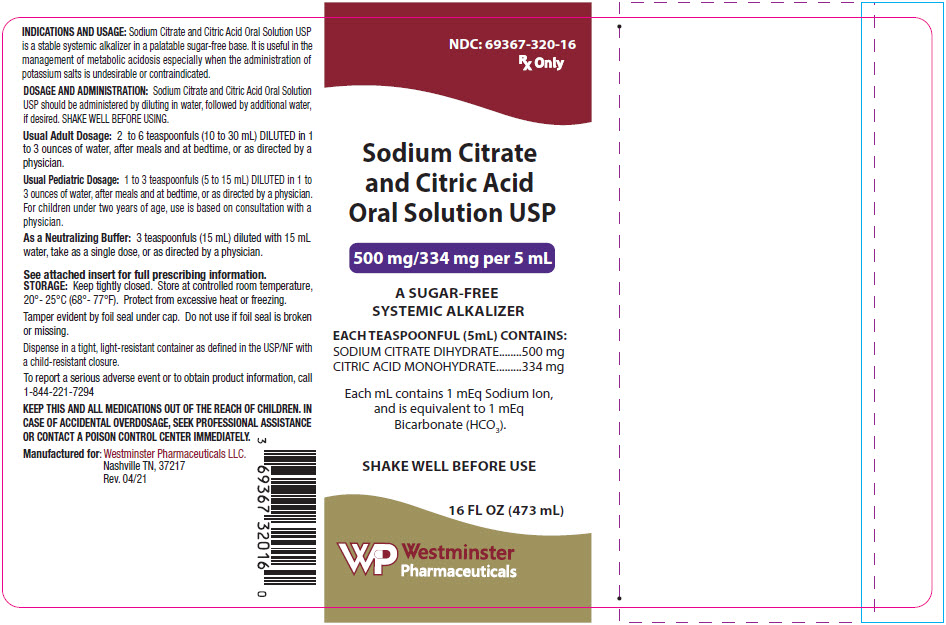
PRINCIPAL DISPLAY PANEL - 473 mL Bottle Label
NDC: 69367-320-16
Rx Only
Sodium Citrate
and Citric Acid
Oral Solution USP
500 mg/334 mg per 5 mL
A SUGAR-FREE
SYSTEMIC ALKALIZER
EACH TEASPOONFUL (5mL) CONTAINS:
SODIUM CITRATE DIHYDRATE
500 mg
CITRIC ACID MONOHYDRATE
334 mg
Each mL contains 1 mEq Sodium Ion,
and is equivalent to 1 mEq
Bicarbonate (HCO3).
SHAKE WELL BEFORE USE
16 FL OZ (473 mL)
Westminster
Pharmaceuticals
| SODIUM CITRATE AND CITRIC ACID
sodium citrate and citric acid monohydrate solution |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Westminster Pharmaceuticals, LLC (079516651) |